Abstract

Oleoresins are a mixture of volatile and nonvolatile components of concentrated forms of wholesome products. Even though there are several reports on the effect of spice or spice components on Alzheimer’s disease, there are no studies on the effect of spice oleoresins. Hence, this study investigates the effect of pepper, chili, and turmeric oleoresins in Alzheimer’s type of cognitive impairment in the rat model. The animals were grouped into six groups with six animals in each. They were (i) normal, (ii) scopolamine, (iii) scopolamine + pepper oleoresin, (iv) scopolamine + turmeric oleoresin, (v) scopolamine + chili oleoresin and (vi) scopolamine + donepezil for 13 days. Learning memory and acquisition memory were evaluated by a Morris water maze, and the locomotor activity was assessed by an actophotometer. Biochemical parameters such as AChE, malondialdehyde, reduced glutathione, glutathione peroxidase, superoxide dismutase, and catalase were studied. The brain histology was also studied. The scopolamine treatment significantly (P < 0.05) elevated the locomotor activity and escape latency time and reduced the time spent in the target quadrant, which was reversed in the case of the oleoresin treatment. Scopolamine-mediated changes in AChE, malondialdehyde, reduced glutathione, glutathione peroxidase, superoxide dismutase, and catalase were improved after the treatment with oleoresins. Among the three oleoresins, chili oleoresin were the most effective in behavioral activity, brain biomarkers, and recovery of antioxidant capacities when compared to the drug treatment. Chili and pepper oleoresins improved the protection against hippocampal damage. These oleoresins can be potent preventive/therapeutic agents against Alzheimer’s disease. This study confirms the effect of spice oleoresins in Alzheimer’s disease condition.
Introduction
Alzheimer’s disease (AD) is the most prevailing neurodegenerative disease associated with old age, which leads to progressive memory loss and cognitive impairement.1 AD is characterized by an intracellular and extracellular plaque of β-amyloid peptide (Aβ) and intracellular tangles of hyperphosphorylated tau protein.2 Another hallmark of AD is the fall off of forebrain cholinergic neurons, and a reduction in the Ach level leads to cognitive/memory impairment.3,4 The mechanism involved in AD includes induction of amyloid plaque deposition, expression of inflammatory mediators, increase in oxidation stress, reduction in steroid hormones, etc.5 Although many studies have been done on AD treatment, a promising intervention for curing the disease remains a challenge. Thus, this present research focuses on the potential of spice oleoresins as a potent neuroprotective agent against AD.
Oleoresin is a mixture of volatile and nonvolatile components. It is a concentrated form of wholesome products and marketed as spice drops due to its total pungency and flavor constituents. The oleoresins can be extracted by solvent extraction of ground spice material with organic solvents such as methanol, ethanol, acetone, ethyl acetate, etc., followed by complete removal of solutions to obtain the oleoresins.6,7
Pepper (Piper nigrum L) belongs to the Piperaceae family and is one of the well-known spices globally. It is commonly used as a household spice as a food additive and condiment. In addition, it is also used in traditional medicine for various medicinal purposes in many countries.8 Piperine is a major alkaloid and a pungent nitrogenous substance present in the pepper fruit. Pharmacological studies on the activity of piperine have reported that it has anti-inflammatory and analgesic effect,9−11 cognitive-enhancing effects,12 cytoprotective effects and antioxidant activity,13 antidepressant effects,14 antiulcer effects,15 etc. It is also reported that piperine has a protective effect on neurodegeneration and cognitive impairment.16
Turmeric (Curcuma longa) is a rhizomatous perennial plant and belongs to the family Zingiberaceae. It is used as a spice and coloring agent and in traditional medicine, particularly in South Asia. Traditionally, it is used for wound healing, inflammation, asthma, high cholesterol, etc. The main active component of turmeric is turmerone oil and water-soluble curcuminoids, including curcumin. The medicinal properties of curcumin have been studied, particularly on anticancer activity.17,18 It is also reported that curcumin exhibits antioxidant and anti-inflammatory activities and has potential in a transgenic mouse model in AD.19−22 Curcumin has shown the potential to inhibit the formation of Aβ fibril, and destabilizing the preformed fibril can provide protection in AD and inhibit lipid peroxidation in the rat brain homogenate.23,24 In addition to this, it also inhibits in vitro Aβ fibril formation and protects the cells from Aβ insults.25,26 This supports that turmeric has potential in AD treatment.
Chili (Capsicum annuumL) belongs to the family Solanaceae. It is one of the major spices cultivated worldwide. It has bioactive components with antioxidant activities such as vitamin C, vitamin E, carotenoids, polyphenols, and alkaloids and provides health benefits.27,28 Capsaicin is a major active component of chili, which has the potential for promoting vascular and metabolic health. It was reported that capsaicin has properties like analgesic, antiarthritic, anticancer, and antioxidant properties.29 The study has shown that capsicum oleoresin increases the availability of Ach and suggests that it can be used in the treatment of AD, Parkinson’s disease, myasthenia gravis, ataxia, and senile dementia.30
Although many anti-AD drugs have been approved by the FDA, they have adverse effects such as dizziness, tiredness, nausea, vomiting, heart attack, and stroke. These drugs include AChE inhibitors, anti-inflammatory drugs, receptor antagonists, and monoamine oxidase inhibitors.31 Thus, the prevention of AD with natural products has been gaining interest recently. Considering the above points, this study was aimed to evaluate the effect of spice oleoresins in Alzheimer’s type of cognitive impairment using rat models.
2. Results and Discussion
AD is clinically characterized by a degradation of neurons, decline in memory, and disturbance in neurobehavior. Although many drugs and treatments are available, the severity of the disease has not been under control yet. Therefore, alternative treatments with natural and herbal supplements are rising to manage the AD. Thus, this study was designed to examine cognitive and behavioral functions with the administration of spice oleoresins. A pharmacologically scopolamine-induced AD model has been used widely for the screening of potential cognition-enhancing agents. The improvement in cognitive impairment induced by scopolamine was investigated after the administration of spice oleoresins using behavior and biochemical parameters.
2.1. Effects of Spice Oleoresins on Behavioral Parameters
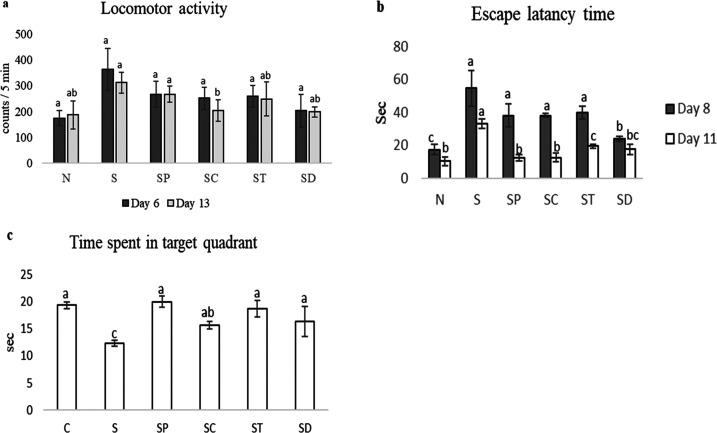
On the 6th and 13th days of the assessment of locomotor activity, the interference in locomotion by the treatment oleoresins and of a drug was ruled out. Scopolamine-administered rats showed higher locomotor activity compared to normal control and oleoresin- and drug-administered rats. Chili-oleoresin- and drug-administered rats showed reduced locomotor activity than pepper- and turmeric-oleoresin administered groups. Pepper- and turmeric-oleoresin-administered groups did not show any notable significant changes in locomotor activity (Figure 1a).
Figure 1.
Locomotor and behavioral parameters. (a) Effects of different oleoresins on the locomotor activity on the 6th and 13th days in a rat model. (b, c) Effects of spices on learning ability (b) and memory (c) in a scopolamine-induced Alzheimer’s-type cognitive impairment in a rat model. C: normal control, S: scopolamine, SP: scopolamine + pepper, SC: scopolamine + chili, ST: scopolamine + turmeric, and SD: scopolamine + drug. All of the values are mean ± standard error of the mean (SEM). Mean values with the same superscript letters are not significantly different, whereas those with the different superscript letters are significantly (P < 0.05) different, as judged by Duncan’s multiple range test.
The escape latency time (ELT) was observed on the 8th to 11th day of the protocol schedule. On the 8th day, there were no significant changes (P < 0.05) found in scopolamine-treated and oleoresin-treated rats, whereas ELT was reduced in donepezil-treated rats and normal rats among the groups. On the 11th day, ELT was decreased in all of the groups. There were no significant changes observed between normal, pepper-treated, and chili-oleoresin-treated groups (P < 0.05). On the 12th day of the protocol schedule, time spent in the target quadrant (TSTQ) was performed, which provided an index of retrieval. Scopolamine-treated rats showed comparatively less TSTQ when compared to normal, oleoresin-treated, and donepezil-treated rats. Although the chili-oleoresin-treated group showed slightly lower TSTQ, there were no remarkable changes between normal, pepper, turmeric oleoresin, and donepezil groups (P < 0.05) (Figure 1b,c). Behavioral activities such as ELT and TSTQ were studied during the acquisition (learning ability) and retrieval trials (memory). It is essential to observe that the Morris water maze (MWM) test investigating spatial learning and memory was used in detecting changes of the central cholinergic system.32 In the MWM test, rats showed a significant decrease in day 4 ELT when compared to day 1 ELT. They showed normal learning ability. In addition, day five assessments resulted in a significant increase in TSTQ when compared to the time spent in other quadrants. They showed normal retrieval capacity. This reveals that scopolamine produced the abnormal learning and memory process in rats. Therefore, in this study, the design administration of pepper and chili oleoresins for 13 consecutive days showed significant attenuation against scopolamine-induced changes in learning and memory dysfunction. However, the turmeric-oleoresin-treated group produce showed less significant improvements compared to other oleoresin-treated groups.
The result of locomotor activity suggests there was no inference or sedative effect in MWM. Thus, ELT and TSTQ in MWM purely resulted in enhanced memory. Therefore, spice oleoresins can improve the long-term memory induced by scopolamine.
2.2. Effects of Spice Oleoresins on Biochemical Parameters
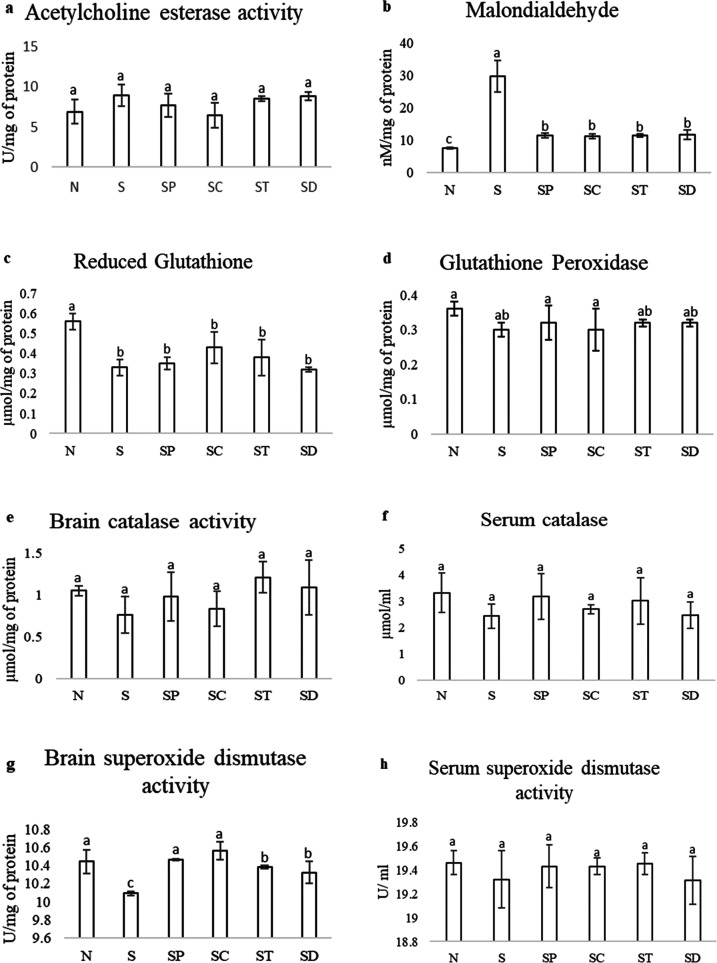
The AChE activity was found to be prolonged in scopolamine-administered rats compared to that in normal animals. The activity was reduced 5–15% in pepper-, turmeric-oleoresin- and drug-administered rats. However, 50% of AChE was reduced in rats administered with chili oleoresin. There was no significant difference (P < 0.05) found among the other groups (Figure 2a). AChE is a major brain biomarker associated with AD. This enzyme is responsible for the degradation of acethylcholine. AChE hydrolyzes ACh to choline and acetate and terminates its effect at cholinergic synapses.33 AChE activity was measured to explore the effect of spice oleoresins on the cholinergic function, which governs vital aspects of memory and other cognitive functions. In this study, scopolamine elevates AChE activity, an enzyme responsible for the deterioration of Ach. The previous research on the inhibitory effect of pepper oil showed that 93% of AChE activity was inhibited, with an IC50 value of 5.97 μg/mL.34 Curcuminoids showed the AChE inhibitory effect, and reported curcuminoids and bisdemethoxycurcumin were able to reduce 60 and 80% of AChE activity, respectively.35 An in vitro study on chili oleoresin has reported that the maximum inhibition (78.3%) of AChE was found at 140 μg/mL.30
Figure 2.
Effects of spice oleoresin on biochemical parameters such as AChE (a); malondialdehyde (b); reduced glutathione (GSH) (c); glutathione peroxide (d); catalase activity in the brain (e) and the serum (f); and superoxide dismutase activity in the brain (g) and the serum (h) in scopolamine-induced Alzheimer’s disease in a rat model. C: normal control, S: scopolamine, SP: scopolamine + pepper, SC: scopolamine + chili, ST: scopolamine + turmeric, and SD: scopolamine + drug. All of the values are mean ± SEM. Mean values with the same superscript letters are not significantly different, whereas those with the different superscript letters are significantly (P < 0.05) different, as judged by Duncan’s multiple range test.
The MDA level indicates the extent of lipid peroxidation in the brain, which is represented in Figure 2b. The scopolamine-administered rats showed increased MDA levels compared to normal, oleoresin-administered, and standard-drug-administered rats. It was found that there was no significant difference between pepper-, turmeric-, chili-oleoresin-, and drug-administered rats, but they showed slightly higher levels than the normal rats. One of the major and important indicators of neurodegeneration of the brain is lipid peroxidation. Membranes of a neuron contain a large amount of long-chain polyunsaturated fatty acids, which help in the transfer of signals by constructing complex structures. Lipids and proteins are the primary target components, which undergo lipid modification by free radicals in neurodegenerative disease.36 There are many reports on protein oxidation and lipid peroxidation, which lead to damage of the membrane integrity, an essential factor in age-related neurodegenerative disease. They are also implicated in the pathogenesis of AD in humans.37,38 In the present study, scopolamine significantly induced peroxidation of lipids and proteins and reduced activities of antioxidants, which indicates an increase in oxidative stress. MDA is a final product of lipid peroxidation, and it is also a measure of free radical generation. The elevation of MDA levels in scopolamine-treated rats indicates the extent of lipid peroxidation in the brain. To evaluate the effect of spice oleoresins on lipid peroxidation in the brain, the MDA level was determined. The MDA level significantly reversed in the case of oleoresin-treated rats, indicating the reduced lipid peroxidation.
In this study, GSH is decreased significantly in scopolamine-treated rats compared to that in normal animals. It was increased in the chili oleoresin group compared to pepper-turmeric-oleoresin-administered rats, as shown in Figure 2c. Similarly, glutathione peroxidase (GPx) is also found to be slightly less in scopolamine-treated rats compared to that in the normal group. There is no significant difference in other groups (Figure 2d). GSH is a cellular antioxidant and provides protection from oxidation stress. It is a tripeptide of glutamic acid, cysteine, and glycine.39 GPx catalyzes the reduction of H2O2 by GSH, which can protect the cells from damage.40,41 The depletion of GSH and GPx in scopolamine-treated rats may be due to the elevation of the MDA level in the brain. GSH is considered as the first-defense endogenous antioxidant against oxidative stress produced by H2O2.36,42 The present study is consistent with previous studies; the GSH and GPx levels decreased with scopolamine administration; further, they were slightly increased with co-administration of spice oleoresins.
In this study, both superoxide dismutase (SOD) and catalase activities were significantly reduced in the brain homogenate as well as in the serum. In the brain homogenate, SOD was increased slightly after the administration of chili and pepper oleoresins (Figure 3a). There was no significant difference found in SOD activity in the serum in all of the groups (Figure 3b). In both the brain homogenate and the serum, the catalase activity was slightly higher in the turmeric and pepper oleoresin groups compared to that in chili-oleoresin- and drug-administered groups. However, there was no significant difference (P < 0.05) found between the group of oleoresins (Figure 3c,d). SOD and catalase are the most important antioxidant enzymes. The SOD detoxifies the superoxide anions, which cause damage to the cell membrane. On the other hand, catalase detoxifies H2O2 radicals, which contribute to the oxidative stress in AD.43 In the present study, the increase in SOD and catalase activities in turmeric and pepper oleoresin groups may be due to the strong antioxidant potential of turmeric and pepper against protein oxidation, glycoxidation, glycation, etc. It was reported that Scopolamine also reduced the catalase level in mice, which was also improved after the treatment with meloxicam and selegiline.44
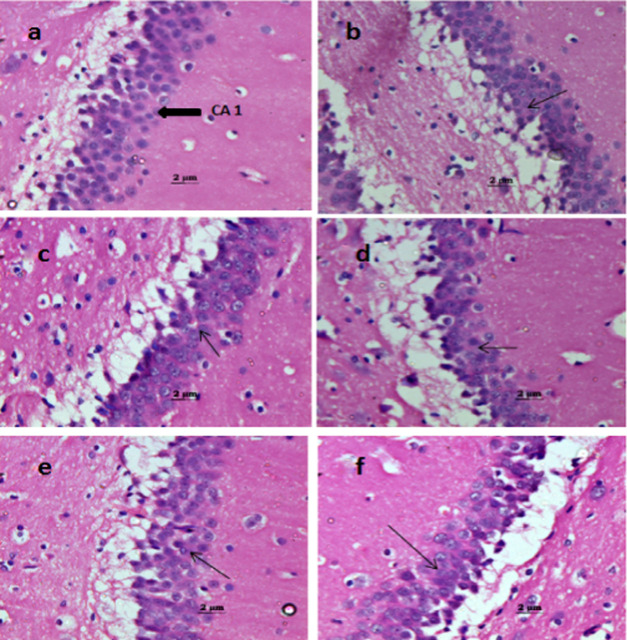
Figure 3.
Effect of spice oleoresins on the brain histology at the CA1 region: (a) control; the normal arrangement of pyramidal cells and polymorphic cells. (b) Scopolamine-treated group; degeneration of pyramidal cells and slight disorganization of the pyramidal layer. (c) Scopolamine + pepper oleoresin; partial repair of the pyramidal layer. (d) Scopolamine + chili oleoresin; significant repair of the degenerated pyramidal layer. (e) Scopolamine + turmeric oleoresin and (f) scopolamine + drug; both groups show attenuation of scopolamine-induced degeneration of cells in Alzheimer’s dementia.
2.3. Effects of Spice Oleoresins on the Histopathology of the Brain
In this study, brain tissue samples were collected and subjected to tissue H&E staining for the illustration of the neuroprotective effect of oleoresins. After 13 consecutive days of scopolamine treatment, neuronal loss, hypoperfusion, and hippocampal atrophy were observed in the hippocampal area. Treatment with spice oleoresins showed an improvement in the hippocampal damage as well as neuroprotection. Among all spice oleoresins, pepper and chili showed a significant effect compared to the turmeric-treated group on neuronal damage. Aggregation of amyloid-beta, the formation of tau protein plaques, apoptosis of neuronal cells, etc., are the essential features in the pathology of the brain during neuronal damage.45 In the present study, spice oleoresin reduced the hippocampal damage and improved the neuroprotection effect.
The results of this study suggest that chili- and pepper-oleoresin-administered rats showed marked improvements in cognitive function when compared to scopolamine-, turmeric-, and drug-administered rats. This study also suggests that spice oleoresins possess neuroprotective effects by scavenging ROS and provide protection against oxidative damage induced by scopolamine. The antioxidant activity and AChE inhibition reveal the contributory effect of spice oleoresins against dementia.
3. Conclusions
Scopolamine induction caused Alzheimer’s-type dementia by increasing the AChE level and oxidative stress, which are majorly associated with cognitive and memory impairments. Based on this study, the major biomarkers like beta-amyloid and inflammatory cytokines can be further evaluated to affirm and uphold the strong evidence of spice oleoresins in the long-term injected-scopolamine-mediated changes. Spice oleoresins improve the antioxidant level and reduce the lipid peroxidation induced by scopolamine compared to drug donepezil and reversed scopolamine-mediated changes. Thus, spice oleoresins could be potential candidates for the treatment of cognitive impairment in AD.
4. Experimental Section
4.1. Spice Oleoresins
Black pepper (Panniyur), turmeric (Alleppey), and red chili (Byadgi) were procured from the Pepper Research Station, Panniyur, Kerala; planters from Alleppey, Kerala; and Byadgi, Karnataka, respectively, and authenticated by a qualified botanist from the University of Mysore, Mysuru, India. All of the spices were cleaned for extraneous materials and powdered in a mechanical grinder and mixer (Philips, India). Each 100 g powdered spice sample was mixed with 300 mL of ethanol (1:3 w/v) in a glass column.The extracts were collected six times at an interval of 1 h. The further solvent was recovered by the flash evaporator (Heidolph Laborota 4000 Efficient, Triad Scientific, Inc. Manasquan). The resin yield was recorded and stored in ambient temperature in dark conditions for further studies. The black pepper, turmeric, and red chili contained 47–50% (w/w), 34–35% (w/w), and 1.7–1.9% (w/w) of piperine, curcumin, and capsaicin, respectively.
4.2. Chemicals
Scopolamine and standards were obtained from Sigma (Bangalore, India), donepezil was obtained from Alkem Laboratories Ltd., Mumbai, India, and other chemicals were obtained from standard chemical suppliers or manufacturers. All of the compounds and solvents were of analytical grade or extrapure.
4.3. Animals and Their Treatment
Male Wistar rats of 280 ± 10 g were obtained from the animal housing facility of the institute (CSIR - Central Food Technological Research Institute, Mysuru - 570020, India). The animals were kept under standard husbandry conditions with food and water. The temperature was maintained at 21 ± 3 °C. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC No.: 160/2019) as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Fisheries, Animal Husbandry and Dairying, Govt. of India, New Delhi. The animals were grouped into six groups with six animals in each. They were (i) normal control, (ii) scopolamine (0.7 mg/kg), (iii) scopolamine (0.7 mg/kg) + pepper oleoresin (50 mg/kg), (iv) scopolamine (0.7 mg/kg) + turmeric oleoresin (50 mg/kg), (v) scopolamine (0.7 mg/kg) + chili oleoresin (50 mg/kg), and (vi) scopolamine (0.7 mg/kg) + donepezil (0.5 mg/kg) for a total of 13 days. The doses were calculated using DoseCal.46 The locomotor activities of the rats were recorded for 2 days (before and after MWM), i.e., 6th and 13th days. The MWM test was performed for 5 days (8th–12th day). On the 14th day, rats were euthanized; the brain and blood samples were collected and analyzed for biochemical parameters.
4.4. Drug Administration
Scopolamine (0.7 mg/kg) was administered by the intraperitoneal route. The oleoresins of the three spices (50 mg/kg) and donepezil (0.5 mg/kg) were administered orally.
4.5. Assessment of Behavioral Activity
4.5.1. Locomotor Activity
The locomotor activity was analyzed by a digital actophotometer (INCO Photo-Actometer, Instruments and Chemicals Pvt. Ltd., Ambala, India). The actophotometer consisted of a closed arena with infrared light-sensitive photocells. Animals were kept inside the closed arena and observed for 5 min. The light beam in the actophotometer cut or crossed by the animals was recorded and expressed as counts per 5 min. The apparatus was kept in a dark, sound-attenuated, and ventilated room to avoid external disturbance, which affects the animal movement.47
4.5.2. Morris Water Maze (MWM)
The MWM evaluates spatial learning and memory. Animals were trained in a circular pool (180 cm diameter and 60 cm depth) with a platform. The pool was divided into four equal quadrants (Q1, Q2, Q3, Q4). One of the quadrants was used as the starting point (same for all of the trials). The pool was kept in the darkroom and filled with water to a 40 cm depth. A movable platform was placed in the pool below the 2 cm water level to analyze the escape latency time (ELT). Animals were trained on 8th–11th days, and ELT (time taken to locate the hidden platform by the animals) was recorded, which gave the index of learning. The rats were allowed to swim for 2 min to find the platform; if they failed, they were navigated onto the platform and allowed to remain on the platform for 20 s. On the 12th day, the platform was removed and rats were allowed to explore the platform for 90 s. The time spent in the target quadrant (TSTQ) was recorded, which gave the index of retrieval.47
4.6. Blood and Brain Sample Collection
All of the animals were euthanized in a CO2 chamber with a flowmeter and a regulator (AIMS, New York) at the end of the experimental period, and the blood was collected and allowed to clot to obtain the serum. The serum was obtained after centrifugation at 3000 rpm for 15 min and stored at −80 °C for further biochemical analysis. The brain was also harvested and fixed in 10% (v/v) formalin to study histology.
4.7. Preparation of the Brain Homogenate
Animals were sacrificed, and the brain was removed. The brain samples were rinsed with normal saline and homogenized with 0.1 M phosphate buffer (pH 7.4) in a tissue homogenizer. The buffer was added 10 times (w/v) and centrifuged at 10 000 rpm for 10 min. The supernatant was collected in different aliquots for the biochemical assay.47
4.8. Estimation of Biochemical Parameters
4.8.1. Protein Estimation
The protein content was estimated by the Bradford method in the brain tissue.48 The brain homogenate (20 μL) was added to 200 μL of Bradford reagents and incubated for 15 min at 37 °C, and the absorbance was measured at 596 nm in a microplate reader (Spark 10M, Tecan Group Ltd., Switzerland). Bovine serum albumin was taken as the standard (0.1–1 mg). The protein content in the brain homogenate was expressed in mg/μ.
4.8.2. Estimation of Acetylcholine Esterase
The AChE activity was analyzed by the Ellmanmethod.49 The brain homogenate (50 μL), 3 mL of 0.1 M phosphate buffer (pH 8), 0.1 mL of 14 mM acetylcholine iodide, and 0.1 mL of 10 mM 5,5-dithiobis(2-nitrobenzoate) were mixed. The mixture was incubated for 5 min. The increase in the absorbance was recorded for 2 min at a 30 s interval at 412 nm using a spectrophotometer (UV-1800 Shimadzu spectrophotometer, Japan). The activity of AChE was expressed in enzyme unit/mg of the protein.
4.8.3. Estimation of Reduced Glutathione (GSH)
GSH was measured spectrophotometrically. A reaction mixture consisting of 50 μL of the supernatant, 1.1 mL of 0.25 M sodium phosphate buffer (pH 7.4), and 130 μL of 0.04% 5,5-dithiobis(2-nitrobenzoate) was prepared. The final volume of the mixture was made up to 1.5 mL with distilled water, and the absorbance was recorded at 412 nm.50 Results were expressed as μmol/mg of the protein.
4.8.4. Estimation of Glutathione Peroxidase (GPx)
GPx was analyzed by the following method briefly. The supernatant (200 μL) was added to a mixture of 1 mL of 0.4 M phosphate buffer (pH 7.0), 1 mL of 5 mM NaN3, and 1 mL of 4 mM glutathione. The mixture was preincubated for 5 min at 37 °C. Then, 1 mL of 4 mM hydrogen peroxide was added and further incubated for 5 min. The amount of excess GSH was measured by the reported method.51 The results were expressed as μmol/mg of the protein.
4.8.5. Estimation of Catalase
The activity of catalase was measured according to the reported method.52 The reaction mixture consisted of 2.9 mL of 10 mM H2O2 in 50 mM potassium phosphate buffer (pH 7) and 0.1 mL of the supernatant or serum. The rate of decrease in the absorbance was recorded at 240 nm for 3 min. The results were expressed as μmol/mg of the protein.
4.8.6. Estimation of Superoxide Dismutase (SOD)
SOD activity was assayed according to the described method.53,54 Pyrogallol solution (0.1 mL of 2.6 mM) in 10 mM HCL was added to 2.8 mL of 0.1 M potassium phosphate buffer (pH 7.4) and 0.1 mL of the homogenate or serum. The rate of increase in the absorbance was recorded at 325 nm for 3 min. One unit of SOD is the amount of the enzyme required to inhibit 50% of pyrogallol in a 3 mL mixture. The SOD activity was expressed in U/mg of the protein.
4.8.7. Estimation of Malondialdehyde (MDA)
The end product of the lipid peroxidation is MDA. The MDA level in the brain homogenate was determined quantitatively by the method described earlier.55,56 The tissue homogenate (0.2 mL) was mixed with 0.2 mL of 8.1% (w/v) SDS, 1.5 mL of 20% (v/v) acetic acid (pH adjusted to 3.5), and 1.5 mL of 0.8% (w/v) TBA (aqueous solution). The final volume was adjusted to 4.0 mL with distilled water and incubated for 60 min at 95 °C in a water bath. Further, 1 mL of distilled water and 5 mL of a mixture of n-butanol and pyridine (15:1 v/v) were added after cooling. Then the mixture was centrifuged for 10 min at 4000 rpm, and the organic layer was measured at 532 nm. The MDA concentration was expressed as nmol/mg of the protein.
4.9. Statistical Analysis
All of the experiments were done in triplicate, and the data were presented as mean ± standard deviation. ANOVA was performed, and the means were compared using Tukey’s test. P < 0.05 was considered statistically significant.
4.10. Histopathology of the Brain
The brain samples fixed in 10% formalin were further processed and fixed in paraffin blocks to obtain 5 μm sections. The sections were stained with haematoxylin and eosin. The histological sections were observed under a bright-field microscope (BX 51, Olympus microscope, Japan).
Acknowledgments
The authors thanks the Director, CSIR-CFTRI, Mysuru for providing infrastructure to carry out the experiments.
Glossary
Abbreviations Used
- AD
Alzheimer’s disease
- MWM
Morris water maze
- ELT
escape latency time
- TSTQ
time spent in the target quadrant
- GSH
reduced glutathione
- GPx
glutathione peroxidase
- SOD
superoxide dismutase
- MDA
malondialdehyde
Author Contributions
B.B.B. conceptualized and proposed the project. K.R. and S.M. designed and established the conditions for the protocols, carried out the experiment, and prepared the manuscript. M.S.P. supervised the animal experiments.
The authors are grateful to CSIR, New Delhi for funding the mission mode project (HCP0019) for the study.
The authors declare no competing financial interest.
References
- Blennow K.; de Leon M. J.; Zetterberg H. Alzheimer’s disease. Lancet 2006, 368, 387–403. 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- Ballatore C.; Lee V. M. Y.; Trojanowski J. Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Auld D. S.; Kornecook T. J.; Bastianetto S.; Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol. 2002, 68, 209–245. 10.1016/S0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Pimplikar S. W. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2009, 41, 1261–1268. 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R. S.; Lee H. G.; Xiongwei Z.; Perry G.; Smith M. A.; Castellani R. J. Current approaches in the treatment of Alzheimer’s disease. Biomed. Pharmacother. 2008, 62, 199–207. 10.1016/j.biopha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Joy P. P.; Skaria B. P.; Mathew S.; Mathew G.; Joseph A.; Sreevidya P. P.. Lemongrass; Aromatic and Medicinal Plants Research Station: Odakkali. Asamannoor, Kerala, India, 2006; p 32. [Google Scholar]
- Ravindran P. N.; Kallupurackal J. A.. Black pepper. In Handbook of Herbs and Spices; Woodhead Publishing, 2012; pp 86–115. [Google Scholar]
- Jung B. S.; Shin M. K. Encyclopedia of illustrated Korean natural drugs, Araliaceae. Seoul: Young Lim Sa 1998, 439–443. [Google Scholar]
- Lyras L.; Cairns N. J.; Jenner A.; Jenner P.; Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J Neurochem. 1997, 68, 2061–2069. 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- Emilien G.; Beyreuther K.; Masters C. L.; Maloteaux J. M. Prospects for pharmacological intervention in Alzheimer disease. Arch. Neurol. 2000, 57, 454–459. 10.1001/archneur.57.4.454. [DOI] [PubMed] [Google Scholar]
- Gupta S. K.; Bansal P.; Bhardwaj R. K.; Velpandian T. Comparative anti-nociceptive, anti-inflammatory and toxicity profile of nimesulide vs nimesulide and piperine combination. Pharmacol. Res. 2000, 41, 657–662. 10.1006/phrs.1999.0640. [DOI] [PubMed] [Google Scholar]
- Wattanathorn J.; Chonpathompikunlert P.; Muchimapura S.; Priprem A.; Tankamnerdthai O. Piperine, the potential functional food for mood and cognitive disorders. Food Chem. Toxicol. 2008, 46, 3106–3110. 10.1016/j.fct.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Selvendiran K.; Singh J. P.; Krishnan K. B.; Sakthisekaran D. Cytoprotective effect of piperine against benzo[a]pyrene induced lung cancer with reference to lipid peroxidation and antioxidant system in Swiss albino mice. Fitoterapia 2003, 74, 109–115. 10.1016/S0367-326X(02)00304-0. [DOI] [PubMed] [Google Scholar]
- Lee S. A.; Hong S. S.; Han X. H.; Hwang J. S.; Oh G. J.; Lee K. S.; Lee M. K.; Hwang B. Y.; Ro J. S. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem. Pharm. Bull. 2005, 53, 832–835. 10.1248/cpb.53.832. [DOI] [PubMed] [Google Scholar]
- Bai Y. F.; Xu H. Protective action of piperine against experimental gastric ulcer. Acta Pharmacol. Sin. 2000, 21, 357–359. [PubMed] [Google Scholar]
- Chonpathompikunlert P.; Wattanathorn J.; Muchimapura S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem.Toxicol. 2010, 48, 798–802. 10.1016/j.fct.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B.; Kumar A.; Bharti A. C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [PubMed] [Google Scholar]
- Kamat A. M.; Sethi G.; Aggarwal B. B. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-κB and nuclear factor-κB–regulated gene products in IFN-α–sensitive and IFN-α–resistant human bladder cancer cells. Mol. Cancer Ther. 2007, 6, 1022–1030. 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- Eybl V.; Kotyzova D.; Koutensky J. Comparative study of natural antioxidants–curcumin, resveratrol and melatonin–in cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. 10.1016/j.tox.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Li X.; He R.; Cheng S.; Wenjuan X. Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys. 1989, 14, 175–185. 10.1007/BF02797132. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A.; Basu N.; Ghatak N.; Gujral P. K. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions 1982, 12, 508–515. 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- Lim G. P.; Chu T.; Yang F.; Beech W.; Frautschy S. A.; Cole G. M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. 10.1523/jneurosci.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K.; Hasegawa K.; Naiki H.; Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- Rao M. N. A. Curcuminoids as potent inhibitors of lipid peroxidation. J. Pharm. Pharmacol. 1994, 46, 1013–1016. 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Kim H.; Park B. S.; Lee K. G.; Choi C. Y.; Jang S. S.; Kim Y. H.; Lee S. E. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- Kim D. S.; Park S. Y.; Kim J. Y. Curcuminoids from Curcuma longa L.(Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from βA (1–42) insult. Neurosci. Lett. 2001, 303, 57–61. 10.1016/S0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- Dall’Acqua S. Plant-derived acetylcholinesterase inhibitory alkaloids for the treatment of Alzheimer’s disease. Bot.: Targets Ther. 2013, 3, 19–28. 10.2147/BTAT.S17297. [DOI] [Google Scholar]
- Kulisic-Bilusic T.; Katalinic V.; Dragovic-Uzelac V.; Ljubenkov I.; Krisko A.; Dejanovic B.; Jukic M.; Politeo O.; Pifat G.; Milos M. Antioxidant and Acetylcholinesterase Inhibiting Activity of Several Aqueous Tea Infusions in vitro. Food Technol. Biotech. 2008, 46, 368–375. [Google Scholar]
- Prasad B. C. N.; Shrivastava R.; Ravishankar G. A. Capsaicin. Evidence-Based Integrative Medicine 2005, 2, 147–166. 10.2165/01197065-200502030-00006. [DOI] [Google Scholar]
- Priyadarshini M.; Anitha R.; Lakshmi T. Acetylcholinesterase inhibitory effect of capsicum oleoresins–an in vitro study. Int. J. Res. Pharm. Sci. 2018, 9, 1137–1140. [Google Scholar]
- Kannappan R.; Gupta S. C.; Kim J. H.; Reuter S.; Aggarwal B. B. Neuroprotection by spice-derived nutraceuticals: you are what you eat!. Mol. Neurobiol. 2011, 44, 142–159. 10.1007/s12035-011-8168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R.; De Deyn P. P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001, 36, 60–90. 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Ranjan N.; Kumari M. Acetylcholinesterase inhibition by medicinal plants: A Review. Ann. Plant Sci. 2017, 6, 1640–4. 10.21746/aps.2017.06.003. [DOI] [Google Scholar]
- Lomarat P.; Sripha K.; Phanthong P.; Kitphati W.; Thirapanmethee K.; Bunyapraphatsara N. In vitro biological activities of black pepper essential oil and its major components relevant to the prevention of Alzheimer’s disease. Thai J. Pharm. Sci. 2015, 39, 94–101. [Google Scholar]
- Ahmed T.; Gilani A. H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. 10.1016/j.pbb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Lobo V.; Patil A.; Phatak A.; Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin. Interventions Aging 2007, 2, 219. [PMC free article] [PubMed] [Google Scholar]
- Uttara B.; Singh A. V.; Zamboni P.; Mahajan R. T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin P. The synthesis of glutation during congenital hemolyticanemia with reduced glutathione deficiency. Congenital deficiency in erythrocyte glutathionsynthetase. Nouv. Rev. Fr. Hematol. 1965, 5, 707–720. [PubMed] [Google Scholar]
- Mills G. C. Hemoglobin catabolism I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J. Biol. Chem. 1957, 229, 189–197. 10.1093/oxfordjournals.jbchem.a127498. [DOI] [PubMed] [Google Scholar]
- Flohe L. Glutathione Peroxidase: Enzymology and Biological Aspects. Klin. Wochenschr. 1971, 49, 669–683. 10.1007/BF01487101. [DOI] [PubMed] [Google Scholar]
- Younes M. A.; Siegers C. P. Mechanistic aspects of enhanced lipid peroxidation following glutathione depletion in vivo. Chem. Biol. Interact. 1981, 34, 257–266. 10.1016/0009-2797(81)90098-3. [DOI] [PubMed] [Google Scholar]
- Mann H.; McCoy M. T.; Subramaniam J.; Van Remmen H.; Cadet J. L. Overexpression of superoxide dismutase and catalase in immortalized neural cells: toxic effects of hydrogen peroxide. Brain Res. 1997, 770, 163–168. 10.1016/S0006-8993(97)00768-3. [DOI] [PubMed] [Google Scholar]
- Goverdhan P.; Sravanthi A.; Mamatha T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int. J. Alzheimers Dis. 2012, 2012, 1–8. 10.1155/2012/974013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Li C. J.; Lu Y.; Zong X. G.; Luo C.; Sun J.; Guo L. J. Baclofen mediates neuroprotection on hippocampal CA1 pyramidal cells through the regulation of autophagy under chronic cerebral hypoperfusion. Sci. Rep. 2015, 5, 14474 10.1038/srep14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhavi P.; Divyashree S.; Sanjailal K. P.; Muthukumar S. P. DoseCal: a virtual calculator for dosage conversion between human and different animal species. Arch. Physiol. Biochem. 2019, 1–5. 10.1080/13813455.2019.1687523. [DOI] [PubMed] [Google Scholar]
- Kaur R.; Mehan S.; Khanna D.; Kalra S. Ameliorative treatment with ellagic acid in scopolamine induced Alzheimer’s type memory and cognitive dysfunctions in rats. Austin J. Clin. Neurol. 2015, 2, 2381–9154. [Google Scholar]
- Khatri D. K.; Juvekar A. R. Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson’s disease. Pharmacol., Biochem. Behav. 2016, 150–151, 39–47. 10.1016/j.pbb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K. D.; Andres V.; Featherstone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 889. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fukuzawa K.; Tokumurai A. Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. 10.3177/jnsv.22.405. [DOI] [PubMed] [Google Scholar]
- Sharma M.; Gupta Y. K. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002, 71, 2489–2498. 10.1016/S0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- Aebi H.Catalase. In Methods of Enzymatic Analysis.; Academic Press, 1974; pp 673–684. [Google Scholar]
- Li X. Improved pyrogallol autoxidation method: a reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. 10.1021/jf204970r. [DOI] [PubMed] [Google Scholar]
- Nandi A.; Chatterjee I. B. Assay of superoxide dismutase activity in animal tissues. J Biosci. 1988, 13, 305–315. 10.1007/BF02712155. [DOI] [Google Scholar]
- Moreadith R. W.; Fiskum G. Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal. Biochem. 1984, 137, 360–367. 10.1016/0003-2697(84)90098-8. [DOI] [PubMed] [Google Scholar]
- Reilly C. A.; Aust S. D. Measurement of lipid peroxidation. Curr. Protoc. Toxicol. 2001, 2.4.1–2.4.13. [DOI] [PubMed] [Google Scholar]