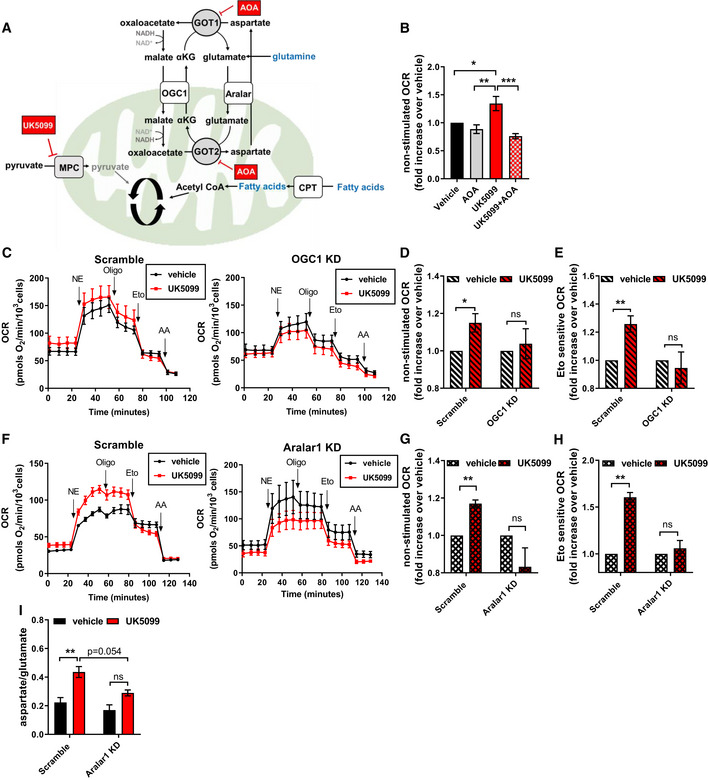
Figure 4. The malate‐aspartate shuttle supports the increase of both glutamine and fatty acid metabolism induced by MPC inhibition.
-
ASchematic representation of proposed mechanism by which MPC inhibition engages the malate‐aspartate shuttle (MASh). GOT1/2, glutamic oxaloacetic transaminase; OGC1, oxoglutarate carrier 1; Aralar, mitochondrial aspartate‐glutamate carrier; CPT, Carnitine Palmitoyltransferase. The coordinated work of OGC1 and Aralar provides a mechanism to allow fatty acid oxidation in the absence of pyruvate import into mitochondria. Fatty acid oxidation generates acetyl‐CoA which condenses with oxaloacetate to generate citrate. In the absence of pyruvate import into mitochondria, oxaloacetate can be generated by glutamine metabolism.
-
BEffect of transaminase inhibition on UK5099‐induced energy expenditure. Brown adipocytes were pre‐treated with vehicle (DMSO), 50–100 nM UK5099, 1 mM aminooxyacetic acid (AOA), or a combination of UK5099 and AOA for 2 h. OCR were measured in respirometry media supplemented with 5 mM glucose and 3 mM glutamine in the presence of the tested compounds. Data show quantification of non‐stimulated OCR from n = 5 individual experiments. Data were normalized to vehicle for each experiment. *P < 0.05, **P < 0.01, ***P < 0.001 by ANOVA.
-
C–EEffect OGC1 downregulation on UK5099‐induced energy expenditure. Primary brown adipocytes were transduced with Scramble RNA (Scramble) or shOGC1 (OGC1 KD) adenovirus. Cells were pre‐treated for 2 h with vehicle (DMSO) or 100 nM UK5099 before OCR measurements. OCR were measured in respirometry media supplemented with 5 mM glucose and 3 mM glutamine in the presence of vehicle or UK5099. Norepinephrine (NE; 1 µM,) oligomycin A (Oligo; 4 µM), etomoxir (Eto; 40 µM), and antimycin A (AA; 4 µM) were injected where indicated. (C) Representative OCR traces averaging 4 technical replicates. (D) Quantification of non‐stimulated OCR as measured in (C) from n = 6 individual experiments. Data were normalized to vehicle for each experiment. ns P > 0.05, *P < 0.05 compared to vehicle by Student’s t‐test. (E) Quantification of Eto‐sensitive OCR as measured in (C) from n = 6 individual experiments. Data were normalized to vehicle for each experiment. ns P > 0.05, **P < 0.01 compared to vehicle by Student’s t‐test.
-
F–HEffect of Aralar1 downregulation on UK5099‐induced energy expenditure. Primary brown adipocytes were transfected with Scramble RNA (Scramble) or siRNA for Aralar1 (Aralar1 KD). Cells were pre‐treated for 2 h with vehicle (DMSO) or 100 nM UK5099 before OCR measurements. OCR were measured in respirometry media supplemented with 5 mM glucose and 3 mM glutamine in the presence of vehicle or UK5099. Norepinephrine (NE; 1 µM), oligomycin a (Oligo; 4 µM), etomoxir (Eto; 40 µM), and antimycin a (AA; 4 µM) were injected where indicated. (F) Representative OCR traces averaging 4 technical replicates. (G) Quantification of non‐stimulated OCR as measured in (G) from n = 4 individual experiments. Data were normalized to vehicle for each experiment. ns P > 0.05, **P < 0.01 compared to vehicle by Student’s t‐test. (H) Quantification of Eto‐sensitive OCR as measured in (F) from n = 4 individual experiments. Data were normalized to vehicle for each experiment. ns P > 0.05, **P < 0.01 compared to vehicle by Student’s t‐test.
-
IBrown adipocytes transfected with Scramble RNA or Aralar1 siRNA were treated for 24 h with vehicle (DMSO) or 10 µM UK5099. Data show quantification of the ratio of aspartate to glutamate abundance as measured by GC‐MS from n = 3 individual experiments. ns P > 0.05, **P < 0.01 by two‐way ANOVA. Note that both carriers of malate‐aspartate shuttle, OGC1 and Aralar1, are required for UK5099‐induced energy expenditure.
Data information: All data are presented as mean ± SEM.
