Abstract
Hyperpigmentation resulting from the overactivation of tyrosinase leads to darker spots or patches on the human skin. Although these phenomena are harmless, there is still great demand for melanogenesis inhibitors to prevent hyperpigmentation by inhibiting the tyrosinase, a rate-limiting enzyme in melanogenesis. Although Lepisorus thunbergianus has been used in folk remedies as a diuretic and hemostatic agent, its effect on melanogenesis has not yet been reported. In this study, we prepared an L. thunbergianus extract and its solvent fractions and evaluated their biological activity against free radical and melanin synthesis. The extract of L. thunbergianus inhibited mushroom tyrosinase activity more efficiently than, and with similar antioxidant activity to, arbutin in vitro. Comparative evaluation of the anti-melanogenesis and anti-tyrosinase activity of L. thunbergianus solvent fractions demonstrated that, by inhibiting tyrosinase activity, the butanol fraction has the highest potential for the inhibition of melanogenesis in melanoma cells. We found by structural analysis using high-performance liquid chromatography (HPLC) and NMR spectroscopy that the major compounds in butanol fraction were three caffeoylquinic acid derivatives. The three derivatives had similar radical scavenging and anti-tyrosinase activities in vitro, while only 5-caffeoylquinic acid had an inhibitory effect on α-MSH-induced melanogenesis. The inhibitory effect of 5-caffeoylquinic acid was verified by the determination of the melanin content and tyrosinase activity in melanoma after treating the cells with a commercial compound. Further, we revealed that 5-caffeoylquinic acid inhibited melanogenesis by chelating a copper cation from a copper–tyrosinase complex. Thus, 5-caffeoylquinic acid or butanol fraction isolated from L. thunbergianus might be useful in cosmetics as a skin-whitening agent.
Introduction
Melanin, a group of natural pigments found in most organisms, plays an important role in the browning of fruits, fungi, and vegetables and the pigmentation in the human skin.1 Melanin is produced from amino acid tyrosine via a multistep process that includes enzymatic oxidation and polymerization.2 In humans, melanin pigment is synthesized by specialized dendritic cell melanocytes located at the junction between the epidermis and dermis in which synthesis is initiated by exposure to ultraviolet (UV) rays.3 Melanin pigment is transported to keratinocytes to protect the skin against UV rays and free radicals; thus, the skin color is determined by the distribution pattern of the melanin pigment.2 Dysregulation in melanin synthesis leads to many forms of hyperpigmentation, such as melasma, freckles, age spots, solar lentigo, post-inflammatory hyperpigmentation, and other hyperpigmentation syndromes.4 More than a hundred proteins are related to melanin synthesis, and among them, tyrosinase is the rate-limiting enzyme that is recognized as being responsible for melanin synthesis.5 Thus, most research works to prevent hyperpigmentation are focused on the identification, isolation, synthesis, and characterization of new potent tyrosinase inhibitors for the prevention of melanogenesis.1,6 A number of tyrosinase inhibitors, such as kojic acid, arbutin, hydroquinone, azelaic acid, ellagic acid, and tranexamic acid, have been popularly used in the cosmetic industry as anti-melanogenesis agents; however, due to the limitations of chemical drugs, which include cytotoxicity and side effects, there is still demand for new materials with improved safety, stability, and efficacy.7
Natural products, including plants, bacteria, and fungi, have become alternative sources for the discovery of efficient skin-whitening ingredients due to their lesser toxicity and better biocompatibility and bioavailability.1,8Lepisorus thunbergianus is a fern belonging to the polypodiaceae, which grows attached to old rocks or the bark of trees and is distributed in the temperate regions of East Asia, such as Korea, Japan, China, Philippines, and Indochina. L. thunbergianus has been used as a diuretic, haemostatic agent and is antitussive in Korean folk remedies. Previous studies reported that an L. thunbergianus extract has topical anti-lipid peroxidation activity in local and antioxidant activity.9 Furthermore, the L. thunbergianus extract showed significant concentration-dependent growth inhibition in oral cancer and colon cancer cells.10 The L. thunbergianus extract may have potential applications in the cosmetic industry because this plant contains various flavonoid compounds and numerous bioactive molecules, which include kercetin 3-methyl ether-glucoside, vitexin, oriental, eriodiol-glucoside, isovitexin, orientin, isorentin, and caffeoylquinic acid.9a However, the effects of the L. thunbergianus extract on melanin synthesis in melanocytes have yet to be elucidated.
In this study, we prepared the L. thunbergianus extract and its solvent fractions via serial fractionation and investigated the inhibitory effects of the solvent fractions on melanin biosynthesis in B16F10 melanoma cells. We identified the butanol (n-BuOH) fraction as a major portion containing the natural inhibitor and further characterized the effects of caffeoylquinic acid derivatives isolated from the L. thunbergianus extracts on the inhibition of melanin biosynthesis.
Results and Discussion
Ethanol Extract of L. thunbergianus Scavenges 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) Radical and Inhibits Tyrosinase Activity
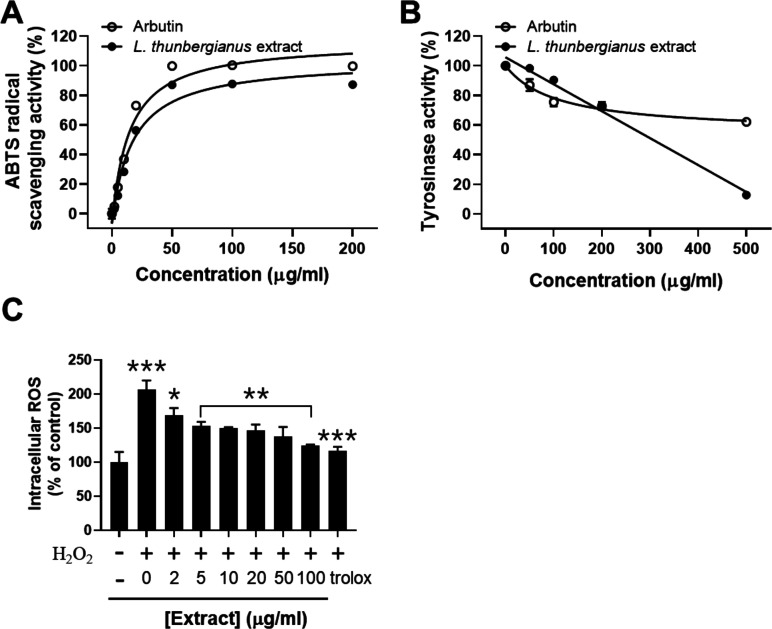
The extraction of L. thunbergianus with 70% ethanol (EtOH) was performed to assess the potential inhibitory activity of natural compounds in the plant. The radical scavenging activity of the L. thunbergianus extract was determined in the concentration range of 2–200 μg/mL. The ethanol extract showed concentration-dependent radical scavenging activity with a maximal effect at 50 μg/mL in which the result was consistent with that of arbutin used as a positive control (Figure 1A).
Figure 1.
Analysis of the L. thunbergianus extract for (A) ABTS radical scavenging, (B) anti-tyrosinase, and (C) intracellular ROS scavenging activities. (A) ABTS radical scavenging activity was determined by measuring the absorbance of the mixture of the ABTS radical and the indicated concentrations of arbutin and the L. thunbergianus extract. (B) Tyrosinase activity was determined via the l-DOPA oxidation assay in the presence of the L. thunbergianus extract at the indicated concentrations. (C) Scavenging activity of the L. thunbergianus extract and trolox on hydrogen peroxide-mediated intracellular ROS was determined using H2DCFDA. Data are expressed as the mean ± SD of three independent experiments.
We also evaluated the inhibitory effect of the extract on mushroom tyrosinase activity by measuring the rate of dopachrome synthesis catalyzed by tyrosinase. The ethanol extract inhibited 87% of tyrosinase activity at 500 μg/mL, while arbutin inhibited only 38% of tyrosinase activity at the same concentration (Figure 1B). Then, we evaluated the scavenging activity of the extract on intracellular ROS increased by 100 μM hydrogen peroxide. Treatment with hydrogen peroxide induced an approximately 2.1-fold increase in the intracellular ROS level of B16F10 cells (Figure 1C). We found that the hydrogen peroxide-mediated increase of intracellular ROS was scavenged by the extract in a dose-dependent manner: the maximal scavenging activity at 100 μg/mL was 78% (Figure 1C). These results suggest that the ethanol extract of L. thunbergianus contains bioactive ingredients that inhibit tyrosinase activity as well as scavenging ABTS radical and intracellular ROS.
Inhibitory Potential of L. thunbergianus Fractions against Melanogenesis
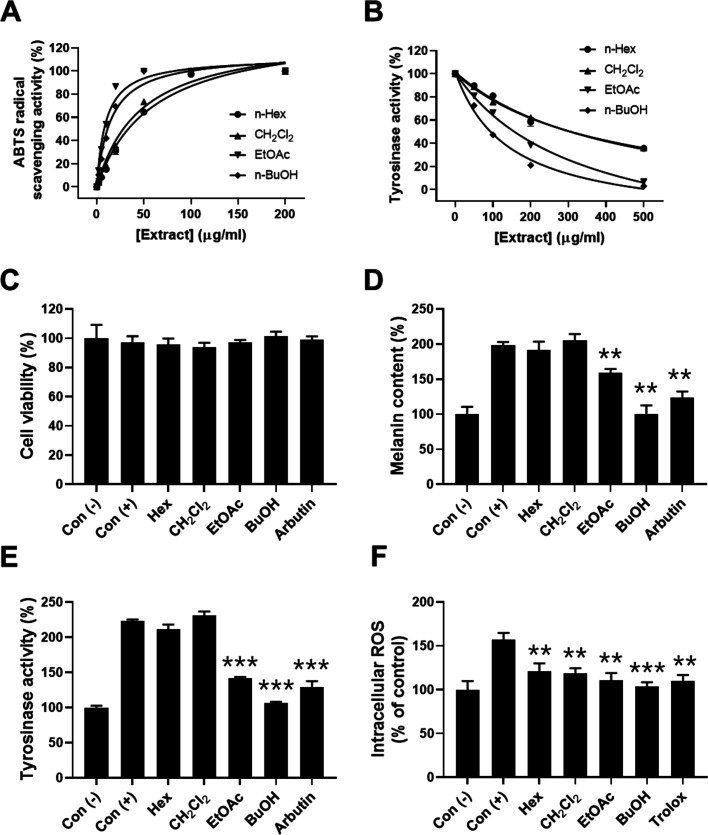
A crude EtOH extract was fractionated with various solvents including hexane (Hex), methylene chloride (CH2Cl2), ethyl acetate (EtOAc), and butanol to identify and characterize ingredients inhibiting melanogenesis. To identify which solvent fractions have the potential against melanin synthesis, we performed comparative analysis of four different L. thunbergianus solvent fractions for the radical scavenging and anti-tyrosinase activities. First, to evaluate the antioxidant activity of the four solvent fractions of the L. thunbergianus extract, we determined the ABTS radical scavenging activities in the concentration range of 2–200 μg/mL. All fractions showed a concentration-dependent increase in ABTS radical scavenging activity (Figure 2A). In particular, EtOAc and n-BuOH fractions completely scavenged the ABTS radical at a 50 μg/mL concentration. The ABTS radical scavenging activity was in the order of n-BuOH > EtOAc > CH2Cl2 > Hex, which is consistent with the previous report.10b Further, the inhibitory effects of different solvent fractions on tyrosinase activity increased in a concentration-dependent manner: the maximal inhibitory activities of n-Hex and CH2Cl2 fractions were below 65%, but those of EtOAc and n-BuOH fractions were above 70% (Figure 2B). The inhibitory activity was in the order of n-BuOH > EtOAc > CH2Cl2 > n-Hex. These results suggest that more hydrophilic fractions, including n-BuOH and EtOAc fractions, showed higher radical scavenging activity and better inhibitory activity than the lipophilic n-Hex and CH2Cl2 fractions.
Figure 2.
Effects of solvent fractions on ABTS radical scavenging, anti-tyrosinase, and anti-melanogenesis activities. (A) ABTS radical scavenging activity was determined by measuring the absorbance of mixtures of the ABTS radical and the indicated concentrations of solvent fractions. (B) Tyrosinase activity was determined by the l-DOPA oxidation assay in the presence of solvent fractions at the indicated concentrations. (C) Cell viability was determined by the MTT assay in the presence of 0.2 mg/mL solvent fractions. (D) Melanin content in B16F10 cells was determined using a photometric method, as described in the Materials and Methods section. (E) Cellular tyrosinase activity was determined by measuring dopachrome resulting from the enzymatic reaction of cell lysates with l-DOPA. (F) Scavenging activity of L. thunbergianus solvent extracts and trolox on hydrogen peroxide-mediated intracellular ROS was determined using H2DCFDA. Data are expressed as the mean ± SD of three independent experiments.
Next, the effects of the L. thunbergianus solvent fractions on melanoma cell proliferation were investigated by treating B16F10 cells with 200 μg/mL each of different fractions or 2 mg/mL arbutin in the presence of an α-melanocyte stimulating hormone (α-MSH), which is well known to promote melanogenesis through microphthalmia-associated transcription factor (MITF) induction.11 The results showed that none of the fractions had any significant effect on melanoma cell proliferation (Figure 2C). We then investigated the inhibitory effect of the solvent fractions on melanogenesis stimulated by α-MSH in melanoma cells. Treatment with α-MSH induced an approximately 2.0-fold increase in the intracellular melanin content of B16F10 cells (Figure 2D). We found that α-MSH-mediated melanogenesis was completely inhibited by the n-BuOH fraction (p < 0.01) and partially inhibited by the EtOAc fraction (40%, p < 0.01), while α-MSH-mediated melanogenesis was not significantly changed by n-Hex and CH2Cl2 fractions (Figure 2D). Further, the inhibitory effects of solvent fractions on tyrosinase activation mediated by α-MSH were determined since tyrosinase plays a key role in melanogenesis. n-BuOH (95%, p < 0.001) and EtOAc (66%, p < 0.001) fractions reversed tyrosinase activation mediated by α-MSH, whereas n-Hex and CH2Cl2 fractions did not, as shown in Figure 2E. Moreover, the scavenging effect of different solvent fractions on the intracellular ROS increase was stimulated by hydrogen peroxide. All solvent fractions reversed the intracellular ROS increase mediated by hydrogen peroxide, as shown in Figure 2F. These results indicate that the BuOH fraction of L. thunbergianus inhibited α-MSH-induced melanin synthesis by inhibiting the intracellular tyrosinase activity in B16F10 cells. Furthermore, these results suggest that the bioactive ingredients inhibiting melanin synthesis were hydrophilic and were mainly found in the n-BuOH fraction.
Identification and Characterization of Bioactive Compounds of L. thunbergianus Extract
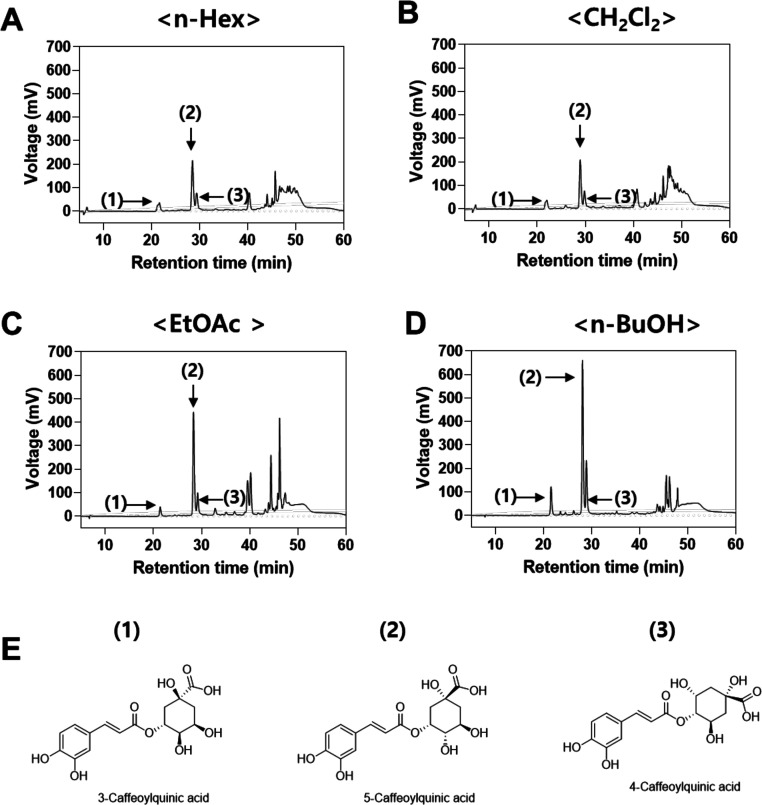
To identify and characterize ingredients inhibiting melanogenesis, high-performance liquid chromatography (HPLC) analysis for all solvent fractions was performed. HPLC analysis of solvent fractions revealed that all fractions contain three major compounds at retention times of 21, 28, and 29 min for compounds 1, 2, and 3, respectively (Figure 3A–D). These three major compounds were further isolated by preparative HPLC purification. The amounts of the three major compounds in the n-BuOH fraction were determined using commercial caffeoylquinic acid derivatives as internal standard molecules, according to the previous report.12
Figure 3.
HPLC chromatograms of (A) n-Hex, (B) CH2Cl2, (C) EtOAc, and (D) n-BuOH fractions of L. thunbergianus and (E) structure of the three major compounds.
The isolated compounds were then identified by comparison of their 1H and 13C NMR data with the literature and were found to be in agreement with the proposed structures (Figure 4).13Figure 3E shows the molecular structure of the three compounds. Compound 1 (yield, 3.2 ± 0.1 mg/100 mg n-BuOH fraction) was obtained as a pale yellow powder. 1H NMR (400 MHz, DMSO-d6) δ 9.66 (1H, brs), 9.20 (1H, brs), 7.49 (1H, d, J = 15.9 Hz), 7.04 (1H, s), 6.99 (1H, d, J = 8.2 Hz), 6.78 (1H, d, J = 8.1 Hz), 6.23 (1H, d, J = 15.9 Hz), 5.20 (1H, m), 5.08 (1H, brs), 4.89 (1H, brs) 4.13 (1H, m), 3.84 (1H, m), 3.56 (1H, s), 3.35 (1H, s), 1.99 (1H, m), 1.92 (1H, m), 1.89 (1H, m), 1.79 (1H, m). 13C{1H} NMR (100 MHz, DMSO-d6) δ (176.42, 168.10, 148.14, 145.53, 144.75, 125.65, 121.07, 115.75, 114.71, 114.22, 72.88, 71.01, 70.85, 69.49, 35.22, 33.75). Compound 1 was identified as 3-caffeoylquinic acid by NMR analysis and by comparison with the previous literature.13b
Figure 4.
1H NMR spectra of (A) 3-caffeoylquinic acid, (B) 4-caffeoylquinic acid, and (C) 5-caffeoylquinic acid and 13C NMR spectra of (D) 3-caffeoylquinic acid, (E) 4-caffeoylquinic acid, and (F) 5-caffeoylquinic acid.
Compound 2 (yield, 14.1 ± 0.1 mg/100 mg n-BuOH fraction) was obtained as a pale yellow powder. 1H NMR (400 MHz, DMSO-d6) δ 9.64 (1H, brs), 9.20 (1H, brs), 7.52 (1H, d, J = 15.9 Hz), 7.06 (1H, s), 7.02 (1H, d, J = 8.1 Hz) 6.79 (1H, d, J = 7.9 Hz), 6.30 (1H, d, J = 15.9 Hz), 4.85 (1H, brs), 4.70 (1H, d, J = 4.9 Hz), 4.12 (1H, brs), 3.95 (1H, m), 1.97 (2H, m), 1.88 (1H, m), 1.83 (1H, m). 13C{1H} NMR (100 MHz, DMSO-d6) δ (176.02, 166.27, 148.28, 145.55, 144.77, 125.54, 121.19, 115.76, 114.68, 114.57, 76.66, 73.92, 66.05, 64.31, 48.57, 38.20). Compound 2 was identified as 5-caffeoylquinic acid by NMR analysis and by comparison with the previous literature.13c
Compound 3 (yield, 3.9 ± 0.2 mg/100 mg n-BuOH fraction) was obtained as a pale yellow powder. 1H NMR (400 MHz, DMSO-d6) δ 9.64 (1H, brs), 9.20 (1H, brs), 7.52 (1H, d, J = 15.9 Hz), 7.06 (1H, s), 7.02 (1H, d, J = 8.1 Hz) 6.79 (1H, d, J = 7.9 Hz), 6.30 (1H, d, J = 15.9 Hz), 4.85 (1H, brs), 4.70 (1H, d, J = 4.9 Hz), 4.12 (1H, brs), 3.95 (1H, m), 1.97 (2H, m), 1.88 (1H, m), 1.83 (1H, m). 13C{1H} NMR (100 MHz, DMSO-d6) δ (176.02, 166.27, 148.28, 145.55, 144.77, 125.54, 121.19, 115.76, 114.68, 114.57, 76.66, 73.92, 66.05, 64.31, 48.57, 38.20). Compound 3 was identified as 4-caffeoylquinic acid by NMR analysis and by comparison with the previous literature.13a
Inhibitory Potential of Caffeoylquinic Acid Derivatives Isolated from L. thunbergianus against Melanogenesis
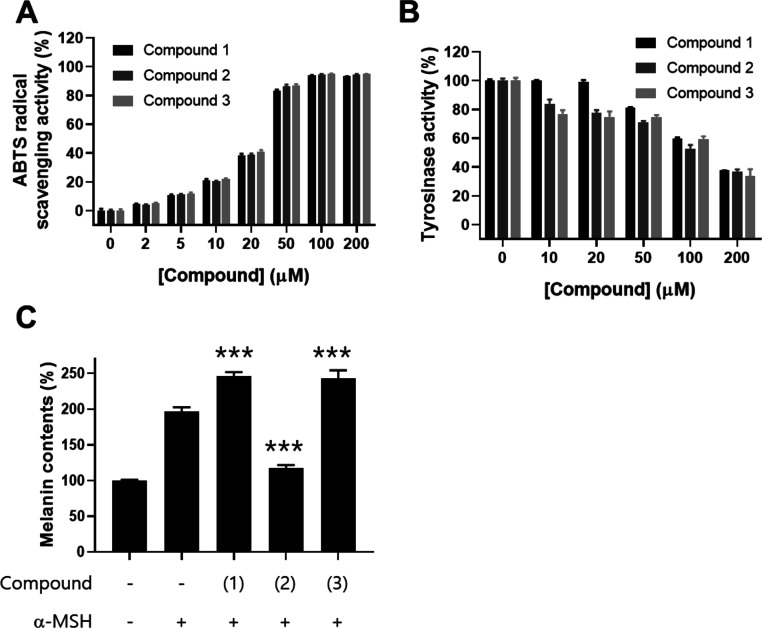
Next, to identify the biologically active ingredient underlying the potent anti-melanogenesis, we determined the inhibitory activity of the three caffeoylquinic acid derivatives isolated from L. thunbergianus against melanin synthesis in melanoma cells. First, the effects of the three derivatives on ABTS radical scavenging were investigated. The results showed that the three derivatives had similar ABTS radical scavenging activities (Figure 5A). We then investigated the inhibitory effect of the three derivatives isolated from L. thunbergianus on tyrosinase activity. The three derivatives showed concentration-dependent inhibition of tyrosinase activity, with maximal inhibition at a 200 μM concentration (Figure 5B). Further, we investigated the inhibitory effect of the three derivatives on α-MSH-induced melanin synthesis in B16F10 cells. Treatment with α-MSH induced an approximately 2.0-fold increase in the intracellular melanin content of B16F10 cells (Figure 5C). Interestingly, compounds 1 (3-caffeoylquinic acid) and 3 (4-caffeoylquinic acid) significantly increased melanin synthesis by 25 and 23%, respectively (Figure 5C, p < 0.001), while compound 2 (5-caffeoylquinic acid) completely reversed α-MSH-induced melanogenesis (p < 0.001). These results suggest that the three caffeoylquinic acid derivatives with similar structures but different substitution sites have different biological activities on melanogenesis. Furthermore, 5-caffeoylquinic acid has a potent inhibitory activity against melanogenesis.
Figure 5.
Effects of the three caffeoylquinic acid derivatives on radical scavenging, in vitro anti-tyrosinase, and anti-melanogenesis activity in B16F10 cells. (A) ABTS radical scavenging activity was determined by measuring the absorbance of mixtures of the ABTS radical and the indicated concentrations of the three derivatives. (B) Tyrosinase activity was determined by the l-DOPA oxidation assay in the presence of the three derivatives at the indicated concentrations. (C) B16F10 cells were treated with 0.1% DMSO as vehicle or with 200 μg/mL of each derivative for 48 h. The melanin content in B16F10 cells was determined using a photometric method, as described in the Materials and Methods section. Data are expressed as the mean ± SD of three independent experiments (***p < 0.001).
Verification of the Anti-Melanogenesis Efficacy of 5-Caffeoylquinic Acid
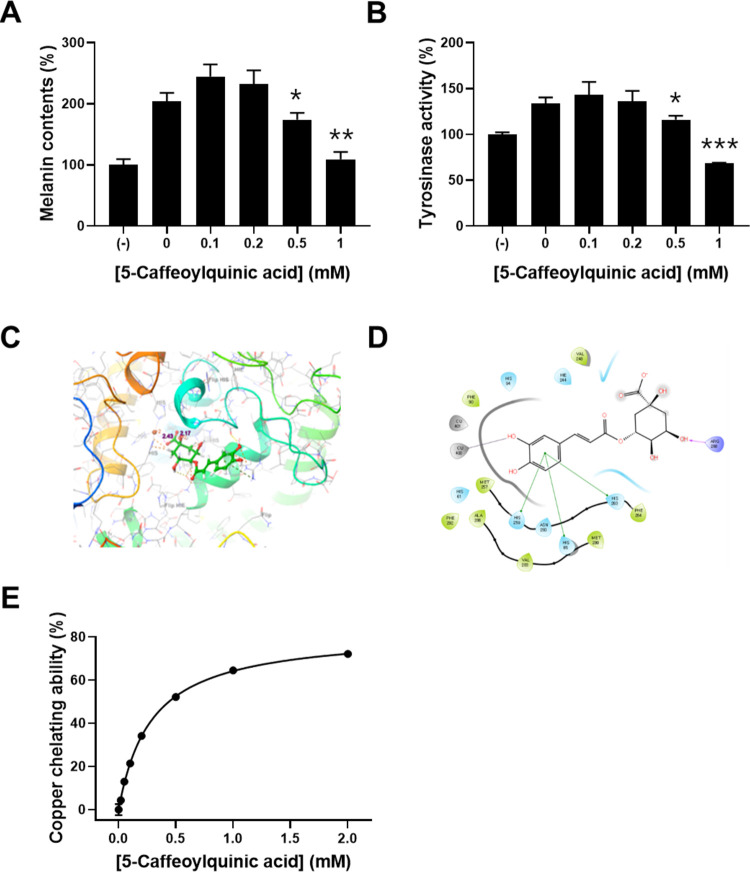
To verify the inhibitory effect of 5-caffeoylquinic acid against melanogenesis, we determined the inhibitory effect of commercial 5-caffeoylquinic acid on melanin synthesis mediated by α-MSH. Treatment with lower concentrations of 0.1 and 0.2 mM 5-caffeoylquinic acid slightly increased melanin synthesis and intracellular tyrosinase activity, while higher concentrations of 0.5 and 1 mM of the compound reversed α-MSH-mediated melanogenesis and intracellular tyrosinase activity (Figure 6A,B) in which the results are consistent with the previous report.14
Figure 6.
Effects of 5-caffeoylquinic acid on intracellular melanin synthesis and its molecular mechanism. B16F10 cells were treated with 0.1% DMSO as vehicle or with 0.1, 0.2, 0.5, and 1 mM 5-caffeoylquinic acid for 48 h. (A) Melanin content in B16F10 cells was determined using a photometric method, as described in the Materials and Methods section. (B) Cellular tyrosinase activity was determined by measuring dopachrome generated by the enzymatic reaction of cell lysates with l-DOPA. (C) Docked pose of 5-caffeoylquinic acid in the binding site of tyrosinase. (D) 2D diagram of the interaction between 5-caffeoylquinic acid and tyrosinase. (E) Copper chelating ability was determined by the pyrocatechol violet assay, as described in the Materials and Methods section. Data are expressed as the mean ± SD of three independent experiments (*p < 0.05, **p < 0.01, ***p < 0.001).
Caffeoylquinic acid is a phenolic compound formed by the ester bond between caffeic acid and l-quinic acid. Caffeoylquinic acid derivatives have been known to have anti-melanogenesis and anti-tyrosinase activities as well as an ROS scavenging activity. Tyrosinase is a multifunctional copper-containing metalloenzyme with divalent copper cations.1 Caffeoylquinic acid derivatives may inhibit tyrosinase activity by chelating a copper cation responsible for sustaining the active state of tyrosinase. To predict the interaction between 5-caffeoylquinic acid and tyrosinase, a computational molecular docking study was performed using the Maestro program. The best pose for the compound, docked to tyrosinase, is depicted in Figure 6C,D. The molecular docking study revealed that 5-caffoylquinic acid interacts with copper ion at a distance of 2.43 Å and establishes a series of π–π stacking with His 259, Asn 260, His 85, and His 263 residues and an H-bond with Arg 268. Furthermore, the binding energy between tyrosinase and 5-caffeoylquinic acid was −4.425 kJ/mol. These results indicate that 5-caffeoylquinic acid could inhibit tyrosinase activity by copper chelation. To verify the inhibitory mechanism of melanogenesis by 5-caffeoylquinic acid, we determined the copper chelating ability of 5-caffeoylquinic acid. The copper chelating ability of 5-caffeoylquinic acid was increased in a concentration-dependent manner (Figure 6E). This result suggests that 5-caffeoylquinic acid inhibits melanin synthesis via chelating a copper cation interacting with tyrosinase.
Materials and Methods
Materials
L. thunbergianus was purchased from the online market www.jirisangol.com (Korea) and dried under ambient conditions before use in this study. Reagents, such as 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), an α-melanocyte stimulating hormone (α-MSH), and enzymes, such as mushroom tyrosinase, were obtained from Sigma-Aldrich (St. Louis, MO, USA). Potassium persulfate (K2S2O8), pyrocatechol violet, and 3,4-dihydroxy-l-phenylalanine (l-DOPA) were purchased from Alfa Aesar (Haverhill, MA, USA).
Extraction and Fractionation of L. thunbergianus
Dried L. thunbergianus (80 g) was pulverized using a pulverizer (HBL-3500S, Samyang Electronics, Korea) and extracted with 70% EtOH three times (800 mL, each time) at 70 °C for 3 days. The resulting extract was vacuum-filtered using Advantec filter paper nos. 1 and 2 (Toyo Roshi Kaisha, Ltd., Tokyo, Japan) and concentrated using a Hei-Vap Advantage rotary evaporator (Heidolph, Germany) to obtain 17.2 g of the EtOH crude extract. This crude extract was suspended in dH2O (180 mL) and successively fractionated with Hex, CH2Cl2, EtOAc, and n-BuOH to yield Hex (1.51 g, 8.8 %), CH2Cl2 (0.88 g, 5.1 %), EtOAc (3.95 g, 23.0 %), and n-BuOH (3.02 g, 17.6 %), as previously described.15 The resulting extracts and fractions were freeze-dried and stored at −80 °C prior to use.
Determination of ABTS Free Radical Scavenging Activity
To evaluate the antioxidant activity of L. thunbergianus extracts and its solvent fractions, the ABTS radical scavenging activity was determined, as previously described.16 To generate the ABTS radical, 10 mL of 7 mM ABTS was mixed with 176 μL of 140 mM potassium peroxydisulfate in dH2O and incubated in the dark at room temperature (RT) for 16 h prior to use. The ABTS radical solution was diluted with absolute methanol to obtain an absorbance of near 0.7 at 734 nm. Aliquots of 100 μL of each extract or fractions in the indicated concentration range of 2–200 μg/mL were added to 100 μL of the diluted ABTS radical solution and incubated for 10 min in the dark at RT. The absorbance was then measured at 732 nm using a SpectraMax M5 multimode microplate reader (Molecular Devices, Sunnyvale, CA, USA). The ABTS radical scavenging activity was calculated as follows
| 1 |
In Vitro Tyrosinase Inhibition
The inhibitory effect of L. thunbergianus extracts and its solvent fractions on tyrosinase activity was assessed by the amount of dopachrome synthesized from the catalytic reaction of tyrosinase.2a,17 Briefly, 50 μL of each extract or fraction in the indicated concentration range was mixed with 50 μL of 50 U/mL mushroom tyrosinase in 50 mM phosphate buffered saline (PBS; 8.1 mM Na2HPO4, 1.2 mM KH2PO4, pH 6.8, 2.7 mM KCl, and 138 mM NaCl) in a 96-well plate and incubated for 30 min at RT. Then, 100 μL of 1 mM l-DOPA was added to each well followed by incubation for an additional 10 min at 37 °C. The absorbance of the resulting solution was measured at 475 nm using the SpectraMax M5 multimode microplate reader.
Cell Culture
B16F10 murine melanoma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Gaithersburg, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco) at 37 °C under humidified 5% CO2.
Cell Viability
The viability of B16F10 cells was determined using an MTT assay, as previously described.18 Briefly, B16F10 cells were seeded in 24-well plates at a density of 1 × 104 cells per well. After 24 h, cells were treated with the indicated concentrations of L. thunbergianus extracts or fractions for 48 h. The cells were then incubated with MTT solution for 4 h, and the reduced formazan crystals were dissolved in DMSO. The resulting solution was transferred to 96-well plates, and the absorbance was measured at 540 nm using the SpectraMax M5 multimode microplate reader.
Determination of Intracellular Reactive Oxygen Species (ROS)
The intracellular ROS level was measured using a DCF/H2DCFDA cellular ROS assay kit (ab113851, Abcam), according to the manufacturer’s instruction. Briefly, B16F10 cells were seeded in 48-well plates at a density of 2 × 104 cells per well. After a 24 h culture, cells were treated for 1 h with the indicated concentrations of L. thunbergianus extracts or solvent fractions in 0.1% bovine serum albumin (BSA) containing culture media with no phenol red. The cells were then incubated with 100 μM hydrogen peroxide for 30 min. After washing with PBS, the cells were treated with 25 μM H2DCFDA in PBS for 45 min. The ROS level was analyzed by a microplate reader (Synergy H1, Biotek, Vermont, USA) equipped with a fluorescence filter at 485/545 nm (excitation/emission wavelength). Average relative fluorescence intensity of control was equated to 100%, with treatment conditions calculated proportionally.
Melanin Content Determination
The melanin content was determined, as previously described, with some modifications.19 The melanoma cells were cultured in a six-well plate for 24 h. They were treated with the indicated concentrations of the L. thunbergianus extract or its solvent fractions for a further 48 h in the presence of 100 nM α-MSH. After washing twice with chilled Dulbecco’s phosphate buffered saline supplemented with calcium chloride and magnesium chloride (D-PBS, Gibco), the resulting cells were detached by incubation with trypsin–EDTA solution. After centrifugation at 1000 rpm for 3 min, the cell pellet was dissolved in 150 μL of 1 M NaOH containing 10% DMSO for 1 h at 60 °C for 1 h. The melanin content was determined by the absorbance at 405 nm using the microplate reader.
Determination of Cellular Tryosinase Activity in Melanoma Cells
Tyrosinase activity in B16F10 cells was examined based on the amount of dopachrome produced from the catalytic reaction of intracellular tyrosinase.20 Briefly, melanoma cells were cultured in a six-well plate for 24 h followed by treatment with different concentrations of the L. thunbergianus extract or its solvent fractions for a further 48 h in the presence of 100 nM α-MSH. After washing twice with ice-cold D-PBS, the cells were lysed in 200 μL of radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich) containing protease and phosphatase inhibitors. After centrifugation of the cell lysate collected from each well at 15,000g for 15 min, 100 μL of the supernatant was mixed with 100 μL of 1 mM l-DOPA in PBS (pH 6.8) followed by incubation for 30 min at 37 °C. The absorbance of dopachrome was measured at 475 nm using the microplate reader. Data were normalized with protein concentration determined by the bicinchoninic acid assay.
Isolation and Characterization of Bioactive Compounds Using HPLC
HPLC was performed on YL-9100 (Young Lin Instrument, Korea), equipped with a TC-C18 column (4.6 mm, 250 mm, and 5 μm, Agilent, USA), in conjunction with a gradient system composed of solvent A (0.2% formic acid) and solvent B (MeOH). The slope solvent ratio was set to 0–5 min, 15% B; 5–10 min, 15–20% B; 10–15 min, 20–30% B; 15–30 min, 30–40% B; 30–37 min, 40–60% B; 37–40 min, 60–100% B; 40–45 min, 100% B; 45–50 min, 100–15% B; and 50–55 min, 15% B. To identify the ingredients in solvent fractions, the mobile phase was delivered at a flow rate of 1 mL/min, and the detection of elute was carried out at 330 nm. To collect the bioactive ingredients in solvent fractions, the mobile phase was delivered at a flow rate of 15.0 mL/min, and the detection of elute was carried out at 330 nm using prep-HPLC (YL-9100 s, Young Lin Instrument, Korea), equipped with a prep-C18 column (4.6 mm, 212 mm, 10 μm, Agilent, USA), in conjunction with a gradient system composed of solvent A (0.2% formic acid) and solvent B (MeOH). The slope solvent ratio was set to 0–10 min, 10% B; 10–20 min, 10–15% B; 20–40 min, 15% B; 40–60 min, 15–20% B; and 60–70 min, 30% B.
Molecular Modeling
The crystal structure of mushroom tyrosinase for molecular modeling was an Agaricus bisporus tyrosinase (PDB dose: 2Y9X) obtained from the Protein Data Bank (PDB). The enzyme was prepared by using the protein wizard preparation workflow embedded in the Maestro program (Maestro, version 11.9.011, Schrödinger, LLC, New York, NY, USA, 2019). Water and all the other molecules present in the pdb files were removed. Molecular docking was performed using the induced fit docking (IFD) protocol (Schrödinger Suite 2019 Induced Fit Docking protocol), as previously reported.21
Copper Chelating Ability
The copper chelating ability of the compounds isolated from L. thunbergianus was determined according to the previous report.22 Each caffeoylquinic acid derivative (10 μL) in the indicated concentration was mixed with 280 μL of 50 mM sodium acetate buffer (pH 6.0), 6 μL of 4 mM pyrocatechol violet solution prepared in the same buffer, and 10 μL of 1 μg/μL CuSO4·5H2O. The disappearance of the blue color was observed by measuring the absorbance at 632 nm using the SpectraMax M5 multimode microplate reader. Water was used as a control instead of a sample. Percent copper chelating activity was calculated from an absorbance at 632 nm, which is as follows
| 2 |
Statistical Analysis
All data in this study were expressed as the mean ± standard deviation (SD) from three independent experiments. Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA, USA). The differences between the mean values of the control and the exposed groups were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. The threshold for statistical significance for all analyses was p < 0.05 (two-tailed).
Conclusions
In this study, we demonstrated that the L. thunbergianus extract scavenges ABTS radicals and exhibits a potent inhibitory effect on melanin biosynthesis without significant cytotoxicity. A bioactive ingredient existing in L. thunbergianus and inhibiting melanogenesis was isolated in the n-BuOH fraction. Furthermore, we found that the three caffeoylquinic acid derivatives are major compounds that exist in the n-BuOH fraction and that 5-caffeoylquinic acid is an important ingredient for suppressing melanogenesis in melanoma cells by inhibiting the cellular tyrosinase activity. Here, we isolated a natural compound inhibitor 5-caffeoylquinic acid from the L. thunbergianus extract to prevent hyperpigmentation and revealed its inhibition mechanism that underlies melanogenesis. Thus, our findings suggest that 5-caffeoylquinic acid and the n-BuOH fraction isolated from L. thunbergianus might be useful in cosmetics as skin-whitening agents.
Acknowledgments
This study was supported by a 2019 research grant from the Ministry of SME and Startups (project no. S2752703).
The authors declare no competing financial interest.
References
- Zolghadri S.; Bahrami A.; Hassan Khan M. T.; Munoz-Munoz J.; Garcia-Molina F.; Garcia-Canovas F.; Saboury A. A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jung S.-H.; Kim J.; Eum J.; Choe J. W.; Kim H.-H.; Kee Y.; Lee K. Velutin, an Aglycone Extracted from Korean Mistletoe, with Improved Inhibitory Activity against Melanin Biosynthesis. Molecules 2019, 24, 2549. 10.3390/molecules24142549. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lim J.; Nam S.; Jeong J. H.; Kim M. J.; Yang Y.; Lee M.-S.; Lee H. G.; Ryu J.-H.; Lim J.-S. Kazinol U inhibits melanogenesis through the inhibition of tyrosinase-related proteins via AMP kinase activation. Brit. J. Pharmacol. 2019, 176, 737–750. 10.1111/bph.14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A. Skin pigmentation enhancers. J. Photochem. Photobiol., B 2001, 63, 148–161. 10.1016/s1011-1344(01)00212-3. [DOI] [PubMed] [Google Scholar]
- Chiang H.-M.; Chien Y.-C.; Wu C.-H.; Kuo Y.-H.; Wu W.-C.; Pan Y.-Y.; Su Y.-H.; Wen K.-C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. 10.1016/j.fct.2013.12.032. [DOI] [PubMed] [Google Scholar]
- a Hearing V. J.; Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991, 5, 2902–2909. 10.1096/fasebj.5.14.1752358. [DOI] [PubMed] [Google Scholar]; b Bennett D. C.; Lamoreux M. L. The color loci of mice--a genetic century. Pigm. Cell Res. 2003, 16, 333–344. 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Momtaz S.; Lall N.; Basson A. Inhibitory activities of mushroom tyrosine and DOPA oxidation by plant extracts. S. Afr. J. Bot. 2008, 74, 577–582. 10.1016/j.sajb.2008.02.005. [DOI] [Google Scholar]
- Lee S. Y.; Baek N.; Nam T.-g. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1–13. 10.3109/14756366.2015.1004058. [DOI] [PubMed] [Google Scholar]
- a Cazarolli L. H.; Zanatta L.; Alberton E. H.; Bonorino Figueiredo M. S. R.; Folador P.; Damazio R. G.; Pizzolatti M. G.; Barreto Silva F. R. M. Flavonoids: prospective drug candidates. Mini-Rev. Med. Chem. 2008, 8, 1429–1440. 10.2174/138955708786369564. [DOI] [PubMed] [Google Scholar]; b Tim Cushnie T. P.; Lamb A. J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- a Yang J.; Kwon Y. S.; Kim M. J. Isolation and characterization of bioactive compounds from Lepisorus thunbergianus (Kaulf.). Arab. J. Chem. 2015, 8, 407–413. 10.1016/j.arabjc.2014.11.056. [DOI] [Google Scholar]; b Chung Y. B.; Lee M. K.; Chong C. Effect of butanol extract from Lepisorus thunvergians on the lipid peroxidantion. Tech. papers Kyungsung Uni. 1993, 207–216. [Google Scholar]
- a Chung K.-T.; Wong T. Y.; Wei C.-I.; Huang Y.-W.; Lin Y. Tannins and human health: a review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]; b Yang J.; Kwon Y. S.; Lim J. D.; Yu C. Y.; Kim M. J. Antioxidant and Anticancer Properties of the Extracts from Lepisorus thunbergianus (Kaulf.) Ching. Korean J. Med. Crop Sci. 2015, 23, 324–333. 10.7783/KJMCS.2015.23.4.324. [DOI] [Google Scholar]
- Shim E.; Song E.; Choi K. S.; Choi H.-J.; Hwang J. Inhibitory effect of Gastrodia elata Blume extract on alpha-melanocyte stimulating hormone-induced melanogenesis in murine B16F10 melanoma. Nutr. Res. Pract. 2017, 11, 173–179. 10.4162/nrp.2017.11.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J.; Kang L.; Liu H.; Xiao Y.; Zhang X.; Chen Y. A validated UV-HPLC method for determination of chlorogenic acid in Lepidogrammitis drymoglossoides (Baker) Ching, Polypodiaceae. Pharm. Res. 2012, 4, 148–153. 10.4103/0974-8490.99076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fayek N. M.; Monem A. R.; Mossa M. Y.; Meselhy M. R. New triterpenoid acyl derivatives and biological study of Manilkara zapota (L.) Van Royen fruits. Pharm. Res. 2013, 5, 55–59. 10.4103/0974-8490.110505. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ge L.; Wan H.; Tang S.; Chen H.; Li J.; Zhang K.; Zhou B.; Fei J.; Wu S.; Zeng X. Novel caffeoylquinic acid derivatives from Lonicera japonica Thunb. flower buds exert pronounced anti- HBV activities. RSC Adv. 2018, 8, 35374–35385. 10.1039/C8RA07549B. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kalinowska M.; Bajko E.; Matejczyk M.; Kaczyński P.; Łozowicka B.; Lewandowski W. The Study of Anti-/Pro-Oxidant, Lipophilic, Microbial and Spectroscopic Properties of New Alkali Metal Salts of 5-O-Caffeoylquinic Acid. Int. J. Mol. Sci. 2018, 19, 465. 10.3390/ijms19020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.-R.; Habasi M.; Xie L.-Z.; Aisa H. A. Effect of chlorogenic acid on melanogenesis of B16 melanoma cells. Molecules 2014, 19, 12940–12948. 10.3390/molecules190912940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk E. B.; Jo A. R.; Oh S. I.; Sohn H. S.; Seong S. H.; Roy A.; Choi J. S.; Jung H. A. Anti-Alzheimer’s disease activity of compounds from the root bark of Morus alba L. Arch. Pharmacal Res. 2017, 40, 338–349. 10.1007/s12272-017-0891-4. [DOI] [PubMed] [Google Scholar]
- Re R.; Pellegrini N.; Proteggente A.; Pannala A.; Yang M.; Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Chang T.-S.; Ding H.-Y.; Tai S. S.-K.; Wu C.-Y. Mushroom tyrosinase inhibitory effects of isoflavones isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288. Food Chem. 2007, 105, 1430–1438. 10.1016/j.foodchem.2007.05.019. [DOI] [Google Scholar]
- Purushotham G.; Padma Y.; Nabiha Y.; Venkata Raju R. R. In vitro evaluation of anti-proliferative, anti-inflammatory and pro-apoptotic activities of the methanolic extracts of Andrographis nallamalayana Ellis on A375 and B16F10 melanoma cell lines. 3 Biotech. 2016, 6, 212. 10.1007/s13205-016-0529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-C.; Hsieh W.-Y.; Niu Y.-L.; Chang T.-M. Inhibitory effects of adlay extract on melanin production and cellular oxygen stress in B16F10 melanoma cells. Int. J. Mol. Sci. 2014, 15, 16665–16679. 10.3390/ijms150916665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R.; Zayats M.; Willner I. Dopamine-, L-DOPA-, adrenaline-, and noradrenaline-induced growth of Au nanoparticles: assays for the detection of neurotransmitters and of tyrosinase activity. Anal. Chem. 2005, 77, 1566–1571. 10.1021/ac048691v. [DOI] [PubMed] [Google Scholar]
- Uysal S.; Ugurlu A.; Zengin G.; Baloglu M. C.; Altunoglu Y. C.; Mollica A.; Custodio L.; Neng N. R.; Nogueira J. M. F.; Mahomoodally M. F. Novel in vitro and in silico insights of the multi-biological activities and chemical composition of Bidens tripartita L. Food Chem. Toxicol. 2018, 111, 525–536. 10.1016/j.fct.2017.11.058. [DOI] [PubMed] [Google Scholar]
- Kubglomsong S.; Theerakulkait C.; Reed R. L.; Yang L.; Maier C. S.; Stevens J. F. Isolation and Identification of Tyrosinase-Inhibitory and Copper-Chelating Peptides from Hydrolyzed Rice-Bran-Derived Albumin. J. Agric. Food Chem. 2018, 66, 8346–8354. 10.1021/acs.jafc.8b01849. [DOI] [PMC free article] [PubMed] [Google Scholar]