Abstract
Caspase malfunction in stem cells often precedes the appearance and progression of multiple types of cancer, including human colorectal cancer. However, the caspase‐dependent regulation of intestinal stem cell properties remains poorly understood. Here, we demonstrate that Dronc, the Drosophila ortholog of caspase‐9/2 in mammals, limits the number of intestinal progenitor cells and their entry into the enterocyte differentiation programme. Strikingly, these unexpected roles for Dronc are non‐apoptotic and have been uncovered under experimental conditions without epithelial replenishment. Supporting the non‐apoptotic nature of these functions, we show that they require the enzymatic activity of Dronc, but are largely independent of the apoptotic pathway. Alternatively, our genetic and functional data suggest that they are linked to the caspase‐mediated regulation of Notch signalling. Our findings provide novel insights into the non‐apoptotic, caspase‐dependent modulation of stem cell properties that could improve our understanding of the origin of intestinal malignancies.
Keywords: Dronc, Drosophila caspases, intestinal homeostasis, intestinal progenitor cells, non‐apoptotic caspase functions
Subject Categories: Autophagy & Cell Death, Development & Differentiation, Regenerative Medicine
Non‐apoptotic activation of the initiator caspase Dronc contributes to preserving intestinal progenitor cells in a quiescent, non‐proliferative and undifferentiated state under experimental conditions without epithelial replenishment demand.
Introduction
Caspases are cysteine‐dependent aspartate‐specific proteases responsible for the implementation of apoptosis (Xu et al, 2018). Recently, an emerging body of evidence is also attributing non‐apoptotic functions to these enzymes in a wide variety of cell types (Arama et al, 2020). In particular, sublethal caspase activity has been shown to modulate the proliferation and differentiation of stem cells through mechanism which is still largely unknown (Bell & Megeney, 2017; Baena‐Lopez et al, 2018b). Previous studies have indicated that caspase‐9 deficiency in patients suffering from colorectal cancer appears to stimulate the proliferation of intestinal precursors, whilst compromising their differentiation (Xu et al, 2013). Furthermore, our laboratory has recently uncovered stereotyped patterns of non‐apoptotic caspase activation in Drosophila progenitor cells (Baena‐Lopez et al, 2018a). Therefore, we decided to investigate the origin and biological significance of such caspase activation patterns in the Drosophila intestinal system.
The evolutionary conservation of gene function and the ease of gene manipulation in Drosophila melanogaster have been routinely exploited to uncover many genetic networks and cellular processes connected with human diseases (Miles et al, 2011). Accordingly, important discoveries regarding intestinal stem cell biology and caspases have been obtained using fruit flies (Miguel‐Aliaga et al, 2018). The caspases are expressed as pro‐enzymes that only become fully active after one or more steps of proteolytic processing (Goyal et al, 2000; Srinivasula et al, 2002; Crawford & Wells, 2011; Parrish et al, 2013; Xu et al, 2018; Baena‐Lopez et al, 2018b). In Drosophila, the intrinsic apoptotic programme is initiated by the upregulation of different pro‐apoptotic proteins (Hid, Reaper, Grim and/or Sickle) (Goyal et al, 2000; Christich et al, 2002; Srinivasula et al, 2002; Wing et al, 2002). These proteins counteract the activity on caspases of the Drosophila inhibitors of apoptosis (DIAP‐1 and DIAP‐2) (Steller, 2008; Leulier et al, 2006; Huh et al, 2007; Silke & Meier, 2013). In pro‐apoptotic conditions, the main Drosophila initiator caspase referred to as Dronc (caspase‐2/9 orthologue in mammals) can molecularly interact with Dark‐1 (Apaf‐1), forming a protein complex termed the apoptosome. These events elicit high levels of activation of Dronc (Rodriguez et al, 1999; Muro et al, 2004; Xu et al, 2005; Cheng et al, 2017), and the subsequent enzymatic processing of the effector caspases (Death caspase‐1, DCP‐1 (caspase‐7); the death‐related ICE‐like caspase, drICE (caspase‐3); Death‐associated molecule related to Mch2 caspase, Damm; and the Death executioner caspase related to Apopain/Yama, Decay). Upon activation, effector caspases disrupt all of the essential subcellular structures leading to cell death (Parrish et al, 2013; Baena‐Lopez et al, 2018b).
The Drosophila intestine contains a subset of intestinal stem cells (ISCs), responsible for the renewal of the gut epithelium (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006; Jiang & Edgar, 2011). ISCs upon demand can also differentiate as either intermediate progenitor cells termed enteroblasts (EBs), or fully differentiated secretory cells called enteroendocrine cells (EEs) (Li & Jasper, 2016). The EBs rarely, if ever, divide but can terminally differentiate as mature absorptive cells referred to as enterocytes (ECs) (Zhai et al, 2017). High levels of the Notch pathway components, Delta (Dl) and Supressor of Hairless (Su(H)), are considered cell identity markers of ISCs and EBs, respectively. In addition, these cell types also express the stem cell marker escargot (esg) (Micchelli & Perrimon, 2006). Loss of esg expression and upregulation of nubbin (nub, hereafter Pdm1) facilitate the conversion of EBs into ECs (Tang et al, 2018). The expression of Prospero is the distinctive marker of EE cells that directly derive from ISCs (Lemaitre & Miguel‐Aliaga, 2013; Guo & Ohlstein, 2015; Zeng & Hou, 2015). An abundant body of literature has described the genetic factors controlling the proliferation and differentiation of ISCs into EBs; however, the conversion of EBs into ECs is less characterised, particularly in non‐regenerative conditions without tissue replenishment. The Notch pathway is one of the critical signalling cascades that regulates gut epithelial homeostasis (Zwick et al, 2019). Low levels of Notch signalling promote the self‐renewal of ISCs, whilst elevated Notch activation stimulates their transition into EBs (Hakim et al, 2010; Guo & Ohlstein, 2015; Zwick et al, 2019). High levels of Notch signalling combined with specific transcription factors also promote the entry of EBs into the EC differentiation programme (Kapuria et al, 2012; Guo & Ohlstein, 2015). In tissue damaging conditions, Notch activation can modulate additional signalling pathways, such as JAK‐STAT and BMP signalling, that stimulate EB differentiation (Zhai et al, 2017). Interestingly, caspase malfunction has also been shown to alter the proliferation and differentiation of ISCs in regenerative conditions (Jin et al, 2013). Here, we describe how the non‐apoptotic activity of the initiator Drosophila caspase Dronc limits the number of progenitor cells and their entry into the EC differentiation pathway, under experimental conditions without basal epithelial replenishment. Importantly, these novel non‐apoptotic caspase functions are strongly linked to the caspase‐dependent modulation of Notch signalling.
Results
Adult intestines reared in experimental conditions without homeostatic cellular turnover show a stereotyped non‐apoptotic caspase activation pattern
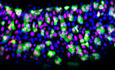
The laboratory developed a caspase sensor to specifically detect the activity of initiator caspases in Drosophila tissues (DBS‐S‐QF sensor) (Baena‐Lopez et al, 2018a). This sensor relies on a truncated form of Drice, attached to the cell membranes at the N terminus. The C terminus contains the transcriptional activator QF (Baena‐Lopez et al, 2018a), which upon initiator caspase‐mediated cleavage, is released and is translocated to the nucleus. The presence of QF in the nucleus can induce the transcriptional activation of any cDNA of interest downstream of the QUAS regulatory sequences (Baena‐Lopez et al, 2018a). Activating cellular markers with variable lifetimes with this tool, we uncovered a stereotyped pattern of non‐apoptotic caspase activation in the progenitor cells of the posterior midgut (R4/5) of adult Drosophila females (Fig 1A) (Baena‐Lopez et al, 2018a). Similar sensors for effector caspases have previously shown reproducible and preferential non‐apoptotic activation in ECs (Appendix Fig S1) (Ding et al, 2016). Following these initial observations, we sought to assess the impact of these caspase activation patterns on intestinal homeostasis. To evaluate the intestinal epithelial status in our experimental conditions, we took advantage of the ReDDM cell lineage‐tracing system (Antonello et al, 2015). This method allows the investigation of intestinal epithelial turnover, through comparing the expression of a short‐lived green fluorescent protein (GFP) and a highly stable Histone protein tagged with a red fluorescent molecule (hereafter Histone‐RFP) (Antonello et al, 2015). This lineage‐tracing tool, under the regulation of the esg‐Gal4 driver, labels undifferentiated intestinal progenitor cells with both fluorescent markers (ISCs and EBs; Fig 1B) (Antonello et al, 2015). However, the silencing of the esg promoter during EB differentiation results in the rapid degradation of the GFP signal, whilst the Histone‐RFP marker persists; the visualisation of Histone‐RFP without GFP can be used as a proxy of differentiation (Antonello et al, 2015). This cell lineage‐tracing method failed to show signs of intestinal regeneration in our experimental conditions (Fig EV1A) during the first 7 days post‐ReDDM induction (Fig 1B and C), and both fluorescent signals overlapped in the intestinal progenitor cells (esg‐positive cells; Fig 1B and C) (Antonello et al, 2015). Despite not observing signs of regeneration, caspases were intriguingly activated in a characteristic pattern under such experimental conditions (DBS‐S‐QF; Fig 1A, Baena‐Lopez et al, 2018a). Expectedly, unspecific tissue damage triggered by either exposure to paraquat [reactive oxygen species (Ali et al, 1996)] or detrimental dietary conditions, caused robust caspase activation (Figs 1D and EV1B) and epithelial regeneration (Figs 1E and F, and EV1C and D); note the presence of newly differentiated ECs with a large nuclear size expressing Histone‐RFP alone (compare Fig 1B with E). These results suggested that our experimental conditions preserve the intestinal epithelia in quiescence state, without cellular death during the first 7‐day post‐ReDDM activation. Supporting this conclusion, the overexpression of the effector caspase inhibitor P35 (Hay et al, 1994) did not cause morphological or cellular alterations in the intestinal epithelium (Fig 1G–J). Collectively, these data indicated the non‐apoptotic nature of DBS‐S‐QF patterns under our experimental regime (Baena‐Lopez et al, 2018a), whilst prompting questions regarding its biological significance.
Figure 1. Non‐apoptotic caspase activation in the Drosophila Intestinal System without the need for tissue replenishment.
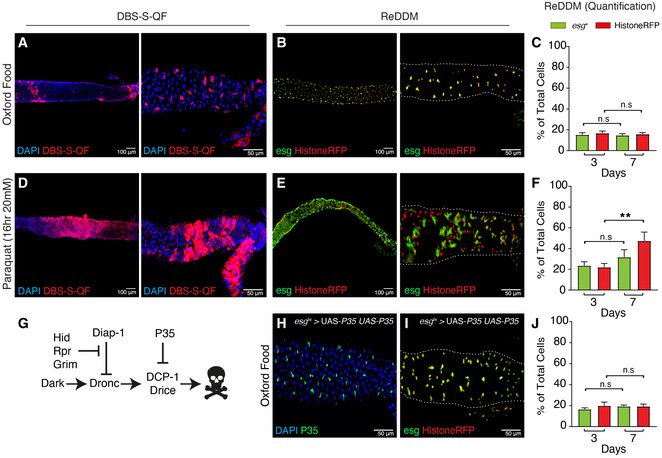
- A representative example of a 7 days (7d) old adult female posterior midgut at low (A) and high magnification (B, Region 4‐Region 5) showing the initiator caspase reporter DBS‐S‐QF (Red, immunostaining anti‐HA). Flies were reared under experimental regime that protects the intestinal epithelial integrity (intestine reared in Oxford Medium and flies transferred every 2 days into vials with fresh food, see Fig EV1) at 25°C. The image on the left was acquired at low magnification with the 10× objective, whilst the right panel was acquired using the 40×. The left and right images in all examples correspond to independent intestines of the same genotype and treatment. Genotype: y1 w1118 UAS‐mCD8::GFP.L QUAS‐mtdTomato‐3xHA Act‐DBS‐S‐QF (BL83131).
- A representative example of a 7‐d intestine reared at 29°C in experimental conditions that protect the epithelial integrity and labelled with ReDDM cell lineage tool. esg (green cytoplasmic signal) labels intestinal progenitor cells, whilst Histone‐RFP (red nuclear signal) acts as a semi‐permanent marker that allows the visualisation of differentiated cells. Note the extensive overlap between the two markers and the absence of differentiated cells only showing the Histone‐RFP labelling, as an indication of negligible epithelial turnover. The image on the left was acquired at low magnification with the 10× objective, whilst the right panel was acquired using the 40×. White dotted line outlines the gut using DAPI staining (not shown) as a reference. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP (Irene Miguel Aliaga)/Cyo; UAS‐Histone‐RFP (BL7019) TubG80ts (BL7019).
- Quantification of intestinal cell subpopulations labelled with ReDDM system at high magnification (GFP and Histone‐RFP) and different time points post‐ReDDM activation (3 and 7 days) in experimental conditions that protect the epithelial integrity; note that none of the cell populations in the gut (GFP (n.s. no significant, P = 0.5267) or Histone‐RFP (n.s., P = 0.2752) significantly increase in number overtime (Quantifications were made using N ≥ 2 biological replicates, total number of guts analysed at 3 days n = 34 and at 7 days n = 45; Mann–Whitney, C). Error bars represent standard error of the mean. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP (Irene Miguel Aliaga)/ Cyo; UAS‐Histone‐RFP (BL7019) TubG80ts (BL7019).
- A representative example of an adult female posterior midgut at low (A) and high magnification (B, Region 4‐Region 5) showing the initiator caspase reporter DBS‐S‐QF (Red, immunostaining anti‐HA) after 16 h of paraquat treatment in Oxford food at 25°C; note the expansion of the labelling with DBS‐S‐QF to large intestinal cells (ECs) (compare D with A). The image on the left was acquired at low magnification with the 10× objective, whilst the right panel was acquired using the 40×. White dotted line outlines the gut using DAPI staining as a reference. Genotype: y1 w1118 UAS‐mCD8::GFP.L QUAS‐mtdTomato‐3xHA Act‐DBS‐S‐QF (BL83131).
- ReDDM lineage‐tracing in an adult intestine reared in Oxford Medium and paraquat (20 mM) during 16 h at 29°C; note the abundance of Histone‐RFP cells without GFP signal, as an indication of epithelial damage and subsequent differentiation of progenitor cells. The image on the left was acquired at low magnification with the 10× objective, whilst the right panel was acquired using the 40×. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP (Irene Miguel Aliaga)/Cyo; UAS‐Histone‐RFP (BL7019) TubG80ts (BL7019).
- Quantification of ReDDM labelling at high magnification after paraquat treatment; note the statistically significant increase (**P = 0.0099) of Histone‐RFP expressing cells without GFP signal (Quantifications were made using N ≥ 2 biological replicates; Unpaired two‐tailed t‐test, 3d n = 9, 7d n = 7). Error bars represent standard error of the mean. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP (Irene Miguel Aliaga)/Cyo; UAS‐Histone‐RFP (BL7019) TubG80ts (BL7019).
- A simplified depiction of the apoptotic pathway in Drosophila. The pro‐apoptotic factors: Hid, Grim and Reaper, stimulate the formation of the apoptosome (Dark and Dronc), resulting in the activation of the effector caspases (Drice & DCP‐1) and cell death. Overexpression of the P35 baculoviral protein inhibits the activity of the effector caspases and cell death.
- Representative example of an intestine 7d old expressing two copies of the effector caspase inhibitor P35 under the regulation of esg‐gal4 (green immunostaining with antibody against P35) at 29°C. Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/ UAS‐P35 (BL5072); UAS‐P35 (BL5073)/+.
- ReDDM lineage‐tracing system in a Drosophila intestine expressing two copies of the effector caspase inhibitor P35 under the regulation of esg‐Gal4 and experimental conditions that protect the epithelial integrity at 29°C. Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/ UAS‐P35 (BL5072); UAS‐Histone‐RFP TubG80ts/ UAS‐P35 (BL5073).
- ReDDM quantification corresponding to the intestines described in (I); no significant increase in either esg (n.s., P = 0.1352) or Histone‐RFP (n.s., P = 0.9801) cell number is observed (Quantifications were made using N ≥ 2 biological replicates; unpaired two‐tailed t‐test, 3d n = 12, 7d n = 11). Error bars represent standard error of the mean. DAPI (blue) labels the nuclei in panels A, D and H. Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/ UAS‐P35 (BL5072); UAS‐Histone‐RFP TubG80ts/ UAS‐P35 (BL5073).
Figure EV1. Schematic of Drosophila experimental regime and results obtained using Drosophila Quick Mix Blue food.
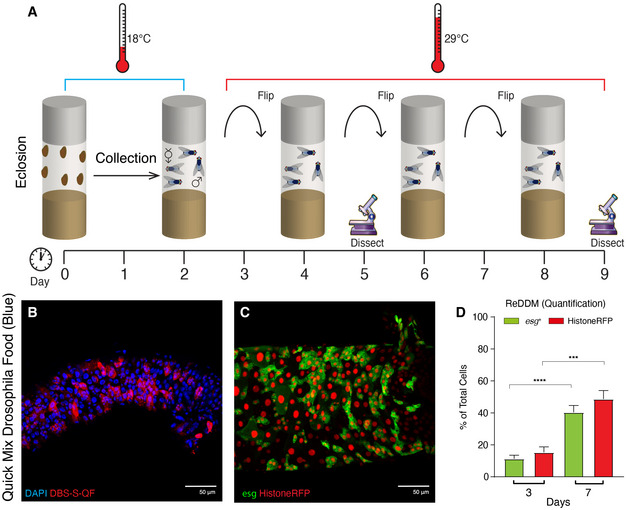
- Parental Drosophila strains were crossed at 18°C and the progeny collected for 2 days post‐eclosion. After 2 days together, adult male and females flies were shifted at 29°C, where they were maintained and transferred to new vials with fresh food every two days. Experimental flies were dissected at 3 and/or 7 days post‐temperature shift at 29°C.
- Representative example of DBS‐S‐QF activation in (red, immunostaining anti‐HA) from flies reared in Drosophila Quick Mix Media (Blue) following the experimental regime described in A; note the widespread activation of the DBS‐S‐QF reporter 7 days post‐temperature shift at 29°C. DAPI (blue) labels the nuclei. Genotype: w1118 DBS‐S‐QF, UAS‐mCD8‐GFP, QUAS‐tomato‐HA.
- Representative example of the ReDDM labelling in a Drosophila intestine reared in Drosophila Quick Mix Media (Blue) following the experimental regime described in A; esg expression (green, GFP) labels intestinal progenitor cells (ISCs and EBs) and Histone‐RFP (red) acts as a semi‐permanent marker of differentiated intestinal cells as either EEs or ECs, after the esg promoter is silenced. Note the high number of Histone‐RFP‐positive cells without GFP signal as an indication of epithelial replenishment. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP/Cyo; TubG80ts UAS‐Histone‐RFP
- Quantification of the ReDDM experiment shown in (C); note the significant increase of esg (****P < 0.0001) and Histone‐RFP (***P = 0.0004) labelled cells (Quantifications were made using N ≥ 2 biological replicates; unpaired two‐tailed t‐test, 3d n = 14, 7d n = 34). Error bars represent standard error of the mean. w1118; esg‐Gal4 UAS‐CD8‐GFP/Cyo; TubG80ts UAS‐Histone‐RFP.
The initiator caspase Dronc prevents unintended differentiation of intestinal progenitor cells
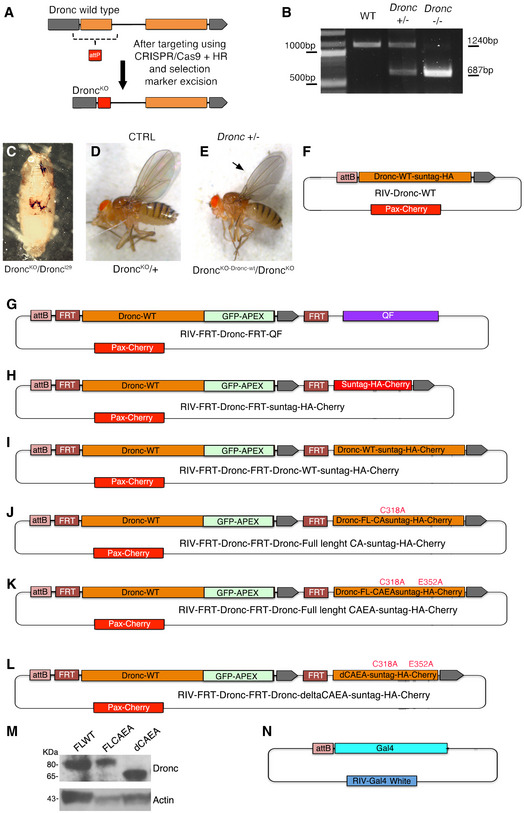
Since P35 only prevents the activity of effector caspases, we investigated the potential implication of initiator caspases in the intestine. To this end, genome engineering protocols (Baena‐Lopez et al, 2013) allowed us to create a new Dronc knockout (Dronc KO) allele that contains an attP‐integration site immediately after the Dronc promoter (Fig EV2A and B). As with the original Dronc null alleles (Chew et al, 2004; Xu et al, 2005), our Dronc KO mutant was lethal during early pupal development, either in homozygosis or in combination of other amorphic alleles (Fig EV2C). Importantly, the heterozygous insertion of a wild‐type Dronc cDNA into the Dronc attP site gave rise to fertile adult flies, similar to their wild‐type siblings (Fig EV2D–F). These results indicated that our rescue construct retained all of the essential functionality of the endogenous gene, whilst validating the attP‐integration site. Next, we created a conditional allele of Dronc (Dronc KO‐FRT Dronc‐GFP‐Apex FRT QF; Fig EV2G) that included a Dronc cDNA flanked by FRT recombination motifs and it was able to rescue the lethality of our Dronc KO mutant. Additionally, this allele had a QF transcriptional activator downstream of the FRT‐rescue‐cassette (Dronc KO‐FRT Dronc‐GFP‐Apex FRT QF). Upon flippase‐mediated excision of the FRT cassette, the production of QF under the transcriptional regulation of Dronc enabled the expression of any QUAS transgene (e.g. QUAS‐LacZ). This feature can be used to identify excision events in Dronc‐transcribing cells and modulate their gene expression profile. Proving the efficiency of our method, 3 days after flippase induction, 91.35% of esg‐labelled cells (GFP‐positive cells) showed the transcriptional activation of the lacZ reporter gene (Appendix Fig S2A and B). Additionally, we noticed signs of hyperplasia and enlarged LacZ‐positive cells without esg expression (GFP‐negative cells) (Appendix Fig S2A). Comparable cellular alterations were observed using a different conditional allele that expressed a suntag‐HA‐Cherry chimeric protein (Dronc KO‐FRT Dronc‐GFP‐Apex FRT Suntag‐HA‐Cherry) (Figs 2A–E and EV2H), thus excluding the association of our original phenotypes with potential QF toxicity (Riabinina & Potter, 2016). Beyond intestinal hyperplasia (Fig 2C and Appendix Fig S2C), the allele conversion using the suntag‐HA‐Cherry construct caused cellular enlargement (Fig 2D and Appendix Fig S2D), and EB differentiation as ECs; note the co‐expression of Histone‐RFP with the EC maker Pdm‐1, and the co‐localisation between the GFP and Pdm1 (inset in Fig 2B and E, Appendix Fig S2E–G). Furthermore, these phenotypes worsened over time (Fig 2C–E). Discarding any detrimental effects linked to the expression of suntag‐HA‐cherry, flies expressing a WT version of Dronc tagged with suntag‐HA‐Cherry (Dronc KO‐FRT Dronc‐GFP‐Apex FRT‐FLWT‐Suntag‐HA‐Cherry) did not display any noticeable intestinal phenotypes (Fig EV3A and F–H, and Appendix Fig S2H). These results were further confirmed inducing loss‐of‐function genetic mosaics of a well‐characterised Dronc null allele (Droncl 29) (Appendix Fig S2I–K); notice the increased number of cells forming Droncl 29 mitotic recombination clones and the presence of large differentiated cells. Importantly, all of these observations were collected in experimental conditions without tissue replenishment and cell death; therefore, we conclude that non‐apoptotic activation of Dronc contributes to preserving the intestinal progenitor cells in quiescence (non‐proliferative and undifferentiated). To further characterise the differentiation status of Dronc mutant intestines, we next analysed the expression profile of metabolic enzymes which are highly enriched in ECs (Doupe et al, 2018). Interestingly, several of these genes were transcriptionally downregulated in our mutant conditions, whilst others were upregulated (Appendix Fig S2L–N). This abnormal gene expression profile of EC markers could be indicative of either premature or partial differentiation of Dronc mutant progenitor cells.
Figure EV2. Genome engineering of the Dronc locus and Dronc alleles.
-
ASchematic diagrams showing the wild‐type configuration of the Dronc locus before (upper lane) and after (bottom lane) targeting with CRISPR/Cas9; note the replacement of the first exon of the gene (orange box) with and attP‐integration site (red box).
-
BAgarose gel showing the PCR amplification of the genomic region around exon 1 of Dronc from larvae of the following genotypes (first lane, Wild‐type +/+), heterozygous mutant flies (second lane, Dronc KO +/−) and homozygous mutant flies (third lane, Dronc KO −/−). Genotypes: (w 1118); (w 1118; Dronc KO/+); (w 1118; Dronc KO/Dronc KO).
-
CThe new Dronc KO‐mutant allele is homozygous lethal in pupal stages in homozygous conditions, but also in trans‐heterozygous combinations with other amorphic alleles (Dronc l29) as shown in the figure. Genotype: w 1118; Dronc KO/Dronc l29.
-
DHeterozygous Dronc KO mutant fly (Dronc KO/+). Genotype: w 1118; Dronc KO/+.
-
EThe insertion of a wild‐type cDNA of Dronc into the Dronc KO allele can rescue at large extent the Dronc insufficiency (Dronc KO‐Dronc‐WT/Dronc KO); notice that only the adult wings were less transparent (arrow) than in the heterozygous controls (d) and sometimes are improperly extended. Genotype: w 1118; Dronc KO/Dronc KO‐FL‐DroncWT‐Suntag‐HA.
-
F–LPlasmid configuration of the different constructs inserted into the Dronc KO attP site.
-
MWestern blot showing the expression of FLWT (Dronc KO‐FLWT‐suntag‐HA‐Cherry/+), FLCAEA (Dronc KO‐FLCAEA‐suntag‐HA‐Cherry/+) and dCAEA (Dronc KO‐dCAEA‐suntag‐HA‐Cherry/+) constructs inserted into the endogenous Dronc locus, note that each of the constructs appear to be expressed at the same levels. Genotypes: (w 1118; Dronc KO‐FLWT‐suntag‐HA‐Cherry/+), (w 1118 Dronc KO‐FLCAEA‐suntag‐HA‐Cherry/+); (w 1118; Dronc KO‐dCAEA‐suntag‐HA‐Cherry/+).
-
NPlasmid configuration of a Gal4 constructs inserted into the Dronc KO attP site.
Figure 2. Effects of Dronc deficiency in intestinal stem cells in experimental conditions without basal cellular turnover.
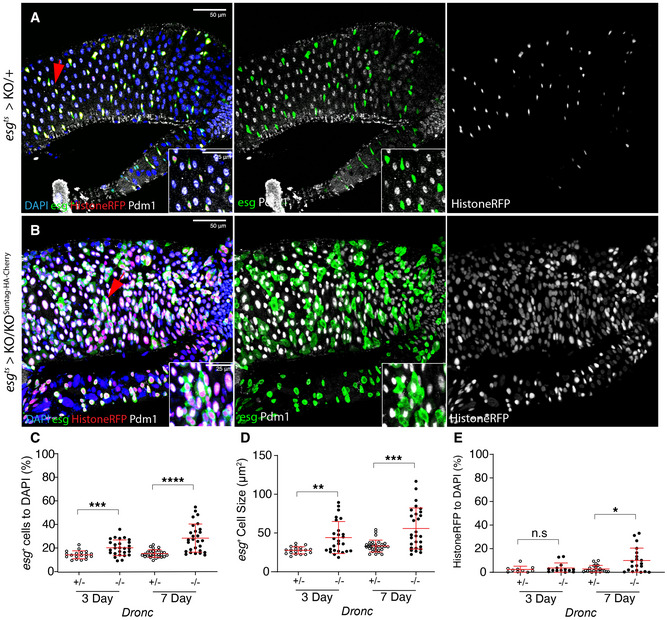
- ReDDM activation in Dronc heterozygous (+/−) intestine 7 days after temperature shift at 29°C; esg expression (green) labels the intestinal progenitor cells, Histone‐RFP (red) is a semi‐permanent marker retained in differentiated cells and Pdm‐1 (grey) labels differentiated ECs. The red arrows indicate the enlarged areas depicted in the insets in the entire figure. DAPI labels the DNA In the entire Figure (blue). All of the experiments in the entire figure have been made under experimental conditions that protect the epithelial integrity (intestine reared in Oxford Medium and flies transferred every 2 days into vials with fresh food). Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO (recombinant chromosome made for this study)/+.
- ReDDM activation in a Drosophila intestine in which all of the progenitor cells are fully mutant for Dronc (see full genotype in Materials and Methods); note the increased cell size of esg‐expressing cells (GFP+, green), the co‐expression of Histone‐RFP (red) and the EC marker Pdm‐1 (grey), and the co‐expression of Pdm‐1 and GFP in enlarged cells (compare panels and detailed insets from A with B). The image is a representative example of a Drosophila adult gut 7 days after temperature shift at 29°C. Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Relative number of esg‐expressing cells normalised to DAPI; notice that the relative percentage of esg‐labelled cells is significantly higher in Dronc fully mutant conditions (−/−) at 3 days (***P = 0.0007) and 7d (****P < 0.0001) post‐temperature shift at 29°C (Quantifications were made using N ≥ 2 biological replicates; unpaired two‐tailed t‐test, +/− n = 32, −/− n = 25). Error bars represent standard deviation of the mean. Genotypes: +/−: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/+; −/−: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Average cell size of esg‐expressing cells (μm2); note the increased cell size of Dronc fully mutant progenitor cells (−/−) at 3d (**P = 0.0014) and 7d (***P = 0.0007) post‐temperature shift at 29°C (Quantifications were made using N ≥ 2 biological replicates; unpaired two‐tailed parametric t‐test, 3d +/− n = 19, 3d −/− n = 28, 7d +/− n = 29, 7d −/− n = 28). Error bars represent standard deviation of the mean. Genotypes: +/−: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/+; −/−: w 1118; esg‐Gal4 UAS‐CD8‐GFP/ +; TubG80ts UAS‐Histone‐RFP Dronc KO/ UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Relative number of esg‐negative cells expressing Histone‐RFP normalised to DAPI; notice that the number of Histone‐RFP cells without esg expression is significantly higher in Dronc fully mutant (−/−) conditions at 7d (*P = 0.0046) (Quantifications were made using N biological replicates ≥2; Mann–Whitney test, +/− n = 24, −/− n = 20. Error bars represent standard deviation of the mean. Genotypes: +/−: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/+; −/−: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
Data information: (A, B) The red arrows indicate the enlarged areas depicted in the insets in the entire figure.
Figure EV3. Dronc functions are related to its enzymatic activity.
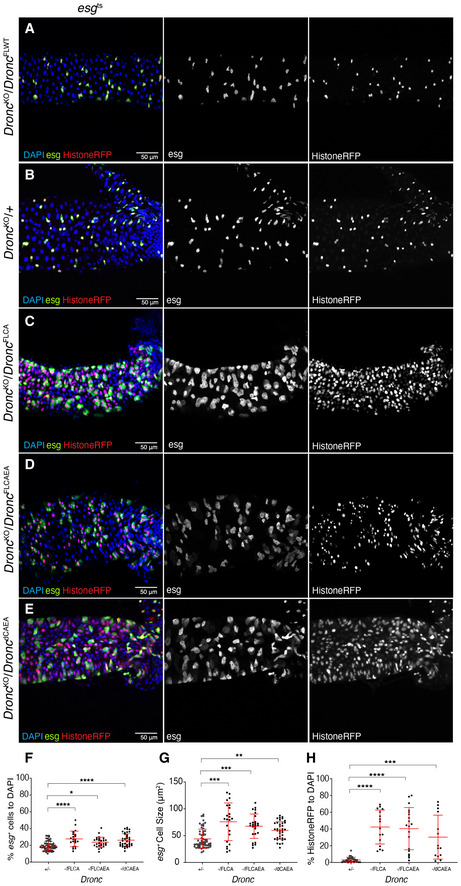
- Representative image of a Dronc WT intestine, reared in Oxford Medium under an experimental regime which protects epithelial integrity, showing ReDDM activation for 7 days post‐temperature shift at 29°C. The Flippase‐mediated excision of the FRT‐flanked wild‐type cDNA of Dronc allows the expression of a wild‐type construct of Dronc tagged with Suntag, HA and cherry that does not cause epithelial alterations. DAPI (blue) labels the nuclei in all the figure. Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase FRT Dronc‐GFP‐APEX FRT Dronc‐FLWT‐suntag‐HA‐Cherry.
- Representative image of a Dronc heterozygous intestine (see full genotype description in Materials and Methods), reared in Oxford Medium under an experimental regime which protects epithelial integrity, showing ReDDM activation for 7 days post‐temperature shift at 29°C; esg expression (green) labels the intestinal progenitor cells, Histone‐RFP (red) is a semi‐permanent marker retained in differentiated cells. Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/+.
- Representative image of ReDDM labelling showing a Dronc‐mutant intestine, expressing a catalytically inactive form of Dronc (FLCA) in progenitor cells (esg‐positive cells, green) in an Dronc KO genetic background for 7 days post‐temperature shift at 29°C; notice that esg‐labelled cells appear enlarged and guts appear hyperplastic compared with (A). Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase FRT Dronc‐GFP‐APEX FRT Dronc‐FLCA‐suntag‐HA‐Cherry.
- Representative image of ReDDM labelling showing a Dronc mutant intestine expressing in progenitor cells (esg‐positive cells, green) a catalytically inactive and non‐cleavable form of Dronc (FLCAEA) in an heterozygous KO Dronc mutant genetic background for 7 days post‐temperature shift at 29°C; notice that esg‐labelled cells appear enlarged and guts appear hyperplastic compared with (A). Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase FRT Dronc‐GFP‐APEX FRT Dronc‐FLCAEA‐suntag‐HA‐Cherry.
- Equivalent to the experiment described in (B) and (C) but in this case esg‐progenitor cells are forced to express a catalytically inactive form of Dronc without the CARD domain (delta‐CAEA, dCAEA). Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase FRT Dronc‐GFP‐APEX FRT Dronc‐delta‐CAEA‐suntag‐HA‐Cherry.
- Quantification of the percentage of esg‐expressing cells relative to DAPI; note the increase of esg‐expressing cells in the different mutant conditions in comparison with a Dronc heterozygous mutant background (FLCA, ****P < 0.0001; FLCAEA *P = 0.0212; ΔCAEA, ****P < 0.0001) (Quantifications were made using N ≥ 2 biological replicates; Dunnett's multiple comparisons test, +/− n = 61, ‐/FLCA n = 21 ‐/FLCAEA n = 26, ‐/ΔCAEA n = 37). Error bars represent standard deviation of the mean. Quantifications in graph refer to genotypes from A–E.
- Quantification of average cell size of esg‐expressing cells (μm2; FLCA, ***P = 0.0002; FLCAEA, ***P = 0.0007; ΔCAEA, **P = 0.0058) (Quantifications were made using N ≥ 2 biological replicates; Dunnett's multiple comparisons test, +/− n = 61, ‐/FLCA n = 23, ‐/FLCAEA n = 26, ‐/ΔCAEA n = 37). Error bars represent standard deviation of the mean. Quantifications in graph refer to genotypes from A–E.
- Quantification of the percentage of Histone‐RFP cells without GFP signal relative to DAPI; notice that the number of cells only expressing Histone‐RFP is significantly higher in the FLCA (****P < 0.0001), FLCAEA (****P < 0.0001) and ΔCAEA (***P = 0.0001) genetic backgrounds (Quantifications were made using N ≥ 2 biological replicates; Dunnett's multiple comparisons test, +/− N = 39, ‐/FLCA n = 16, ‐/FLCAEA n = 19, ‐/ΔCAEA n = 14). Error bars represent standard deviation of the Mean. Quantifications in graph refer to genotypes from A–E.
Dronc functions require its enzymatic activity but are largely independent of the apoptotic cascade
Some of the functions attributed to Dronc rely on protein–protein interactions, instead of its enzymatic activity (Ouyang et al, 2011). Therefore, we decided to investigate the molecular features of Dronc required in our experimental scenario. To this end, we utilised a new set of conditional alleles that expressed enzymatically inactive versions of Dronc upon FRT‐rescue cassette excision (Fig EV2J–L; please see details in the Materials and Methods section). All of these alleles replicated the previously described Dronc‐mutant phenotypes (Fig EV3C–H). Importantly, all of these mutants showed similar expressivity and protein stability, thus discarding any relation of these phenotypes with their differential expression (Fig EV2M). In addition, these findings indicated that the enzymatic activity of Dronc is required for the newly described functions. Next, we explored whether the primary substrates of Dronc during apoptosis, the effector caspases (drIce, Dcp‐1, Decay and Damm), were relevant in our experimental context. Their downregulation failed to replicate the proliferation and differentiation phenotypes observed in Dronc LOF conditions (Fig EV4A–D). Comparable results were obtained downregulating the expression of Dronc‐activating factors, such as Dark and the pro‐apoptotic factors Reaper, Hid and Grim (Fig EV4E–G). These results separate Dronc functions in progenitor cells from the apoptosis programme (see Discussion). They also confirmed the lack of cell death and negligible epithelial turnover in our experimental regime.
Figure EV4. Dronc regulates EB cellular properties independently of the effector caspases and upstream pro‐apoptotic factors.
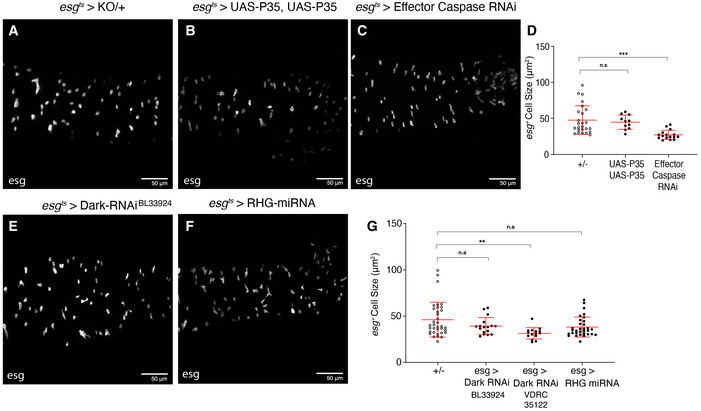
- 7d post‐temperature shift to 29°C. Dronc heterozygous intestine. All of the experiments described in the figure were performed in Oxford medium following an experimental regime which protects epithelial integrity. esg‐Gal4 UAS‐GFP labels ISCs and EBs in the panel A–C, E and F. Genotypes: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP DroncKO/+.
- The overexpression of two copies of the effector caspase activity inhibitor (P35) under the regulation of esg‐Gal4 does not cause morphological defects or an increase in cell size of intestinal progenitor cells (P = 0.8307). Genotypes: w 1118; esg‐Gal4 UAS‐CD8‐GFP/UAS‐P35; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐P35.
- Concomitant overexpression of RNAi against all known Drosophila effector caspases under the regulation of esg‐Gal4. The knockdown of all effector caspases fails to recapitulate the increase in cell size observed following loss of Dronc (P = 0.0002). Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/UAS‐Drice RNAi UAS‐Decay RNAi (Pascal Meier); TubG80ts UAS‐Histone‐RFP DroncKO/UAS‐Damm RNAi UAS‐DCP1 RNAi (Pascal Meier).
- Quantification of average esg cell size (μm2) (n.s. P > 0.5; ***P = 0.001) (Quantifications were made using N ≥ 2 biological replicates; Dunn’s multiple comparisons test, +/− n = 25, 2 × P35 n = 11, Effector Caspase RNAi N = 16). Error bars represent standard deviation of the mean. Quantifications in graph refer to genotypes from A–C.
- The overexpression of RNAi against Dark. There is not significant change in cell size compared with +/− (P = 0.2222). Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Dark RNAi (BL33924).
- Overexpression of a miRNA against the pro‐apoptotic factors. hid, reaper and grim. Note, the inhibition of these factors does not result in any noticeable phenotypes (ns; P = 0.0521). Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐miRNA‐RHG (I. Hariharan).
- Quantification of average esg cell size (μm2). There is no increase in cell size following overexpression of two different Dark RNAis (n.s. P = 0.2222 and **P = 0.0020) or a miRNA against the pro‐apoptotic factors (n.s. P = 0.0521) (Quantifications were made using N ≥ 2 biological replicates; Dunn’s multiple comparisons test, +/− n = 36, esg > Dark RNAi (1) n = 17, esg > Dark RNAi (2) n = 16, esg > RHG miRNA n = 35). Error bars represent standard deviation of the mean. Quantifications in graph refer to genotypes from Fig 4E and F and an extra Dark RNAi (VDRC 35122).
The non‐apoptotic function of Dronc is required in ISCs and EBs
Our previous experiments could not ascribe the novel Dronc functions to a specific gut progenitor cell type. To address this question, we targeted the expression of Dronc in ISCs using the Dl‐Gal4 driver (Zeng et al, 2010). As noted with esg‐Gal4, the excision efficiency of the FRT‐rescue cassette in ISCs using the Dl‐Gal4 line was also very high (81.15%; Appendix Fig S2O and P). However, Dronc deficiency in ISCs did not cause epithelial alterations; no increase of Delta‐positive cells or cell size (Fig 3A–D). We could not observe either a differentiation bias towards EE fate quantifying the number of small nuclei positive for Histone‐RFP and Prospero (Appendix Fig S2Q). Next, we specifically eliminated the expression of Dronc in EBs using the Su(H)‐Gal4 driver (Zeng et al, 2010). Unexpectedly, these experiments also failed to induce significant gut hyperplasia and penetrant differentiation phenotypes (lack of GFP in Su(H)‐expressing cells and retention of Histone‐RFP) in most the analysed intestines (Fig 3E–G). However, the EBs were significantly increased in size (Fig 3H), possibly indicating the initiation of the EC differentiation programme. By comparison with the results obtained using the esg‐Gal4 driver, these new data show that Dronc deficiency appears to sensitise the EBs to differentiate but mainly when its expression is compromised in both progenitor cells (see Discussion).
Figure 3. Phenotypic analysis of Dronc deficiency in escargot‐ and Delta‐expressing cells.
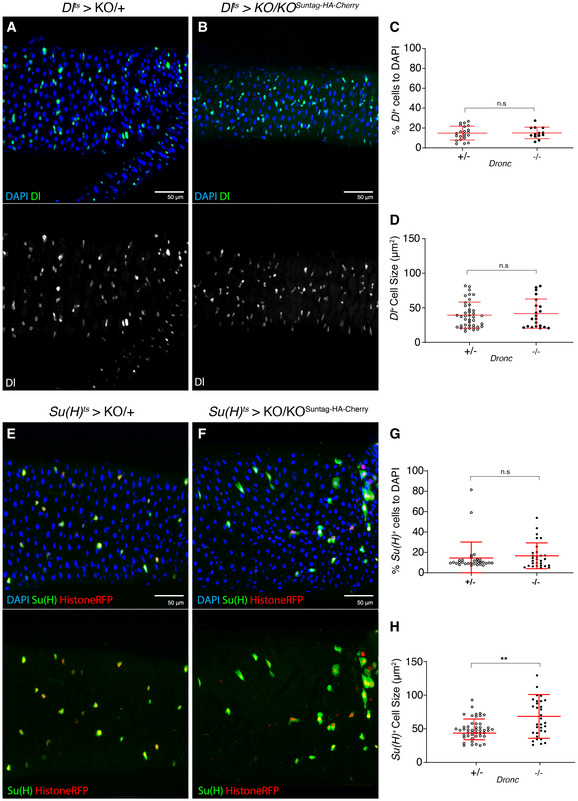
- A 7d intestine Dronc heterozygous (+/−) intestine in which intestinal stem cells express GFP under the regulation of Dl‐Gal4. All of the experiments described in the figure were performed in Oxford medium following an experimental regime that protects the epithelial integrity (flies transferred every 2 days into vials with fresh food). DAPI (blue) labels the DNA in the entire Figure. Genotype: w 1118;;delta‐Gal4 UAS‐GFP TubG80ts UAS‐Histone‐RFP Dronc KO/+ (recombinant chromosome made for this study; the parental line delta‐Gal4 UAS‐GFP TubG80ts UAS‐Histone‐RFP was obtained from Maria Dominguez).
- A 7d intestine in which the Intestinal Stem Cells have become Dronc homozygous mutant (−/−) upon expressing UAS‐Flp under the regulation of Dl‐Gal4. Dl‐expressing cells are labelled with UAS‐GFP (green); there are no morphological differences between (A) and (B). Genotype: w 1118;;delta‐Gal4 UAS‐GFP TubG80ts UAS‐Histone‐RFP Dronc KO/ UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Relative number of GFP + Delta‐expressing cells normalised to DAPI; there is no significant increase in intestinal stem cell number between heterozygous and homozygous Dronc mutant conditions (ns; P = 0.9231) (Quantifications were made using N ≥ 2 biological replicates; unpaired two‐tailed t‐test, +/− n = 22, −/− n = 13). Error bars represent standard deviation of the mean. Genotypes: +/−:w 1118;;delta‐Gal4 UAS‐GFP TubG80ts UAS‐Histone‐RFP Dronc KO/ UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry; −/−: w 1118;;delta‐Gal4 UAS‐GFP TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Average Delta cell size (μm2); notice that the cell size does not change between heterozygous and homozygous Dronc mutant ISCs (ns; P = 0.9694) (Quantifications were made using N ≥ 2 biological replicates; unpaired two‐tailed t‐test, +/− n = 42, −/− n = 21). Error bars represent standard deviation of the mean. Genotypes: +/−:w 1118;;delta‐Gal4 UAS‐GFP TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry; −/−: w 1118;;delta‐Gal4 UAS‐GFP TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- A 7d intestine Dronc heterozygous (+/−) intestine in which intestinal stem cells express GFP under the regulation of Su(H)‐Gal4. Genotype: w 1118; Su(H)‐Gal4 UAS‐GFP; TubG80ts UAS‐Histone‐RFP Dronc KO/+.
- A 7d intestine in which the Enteroblasts have become Dronc homozygous mutant (−/−) upon expressing UAS‐Flp under the regulation of Su(H)‐Gal4. Su(H)‐Gal4‐expressing cells are labelled with UAS‐GFP (green) and UAS‐Histone‐RFP; note the increase in cell size when compared to (E) and the absence of Histone‐RFP cells without GFP signal. Genotype: w 1118; Su(H)‐Gal4 UAS‐GFP; TubG80ts UAS‐Histone‐RFP Dronc KO/ UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Relative number of Su(H)‐Gal4‐expressing cells normalised to DAPI; there is no significant increase in intestinal stem cell number between heterozygous and homozygous Dronc mutant conditions (n.s.; P = 0.5099) (Quantifications were made using N ≥ 2 biological replicates; Mann–Whitney test, +/− n = 30, −/− n = 29). Error bars represent standard deviation of the mean. Genotypes: +/−: w 1118; Su(H)‐Gal4 UAS‐GFP; TubG80ts UAS‐Histone‐RFP Dronc KO/+; −/−: w 1118; Su(H)‐Gal4 UAS‐GFP; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Average Su(H) cell size (μm2), there is a significant increase in Su(H) Dronc null cells (−/−) when compared to the heterozygous control condition (**P = 0.0064) (Quantifications were made using N ≥ 2 biological replicates; Mann–Whitney test, +/− n = 49, −/− n = 33). Error bars represent standard deviation of the mean. Genotypes: +/−: w 1118; Su(H)‐Gal4 UAS‐GFP; TubG80ts UAS‐Histone‐RFP Dronc KO/+; −/−: w 1118; Su(H)‐Gal4 UAS‐GFP; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
Dronc functions rely on its preferential accumulation and activation in progenitor cells
To investigate the molecular origin of the Dronc phenotypes, we analysed the transcriptional activation of Dronc in the posterior midgut, using a newly created Dronc KO‐Gal4 strain (Fig EV2N). This fly line expresses Gal4 under the physiological regulation of the Dronc promoter. Interestingly, Dronc KO‐Gal4 expression was not restricted to the progenitor cells (Appendix Fig S3A). We next investigated the Dronc protein levels in progenitor cells using a published Dronc allele endogenously tagged with the biotin‐ligase TurboID (Shinoda et al, 2019). This enzyme reduces the threshold of detection of any protein of interest by transferring biotin to other proteins in close proximity (Shinoda et al, 2019). Interestingly, this construct revealed a restricted enrichment of biotinylation signal in small progenitor cells under our experimental conditions (Fig 4A). We validated this result taking advantage of a new reporter line created in the laboratory that expresses a catalytically inactive (C318A) tagged form of Dronc under the regulation of the Actin promoter (Appendix Fig S3B). This construct generates fertile adult flies without any noticeable developmental or morphological defects, and the tagging at the C terminus with a modified GFP and Myc facilitates its immunodetection (Appendix Fig S3C). Interestingly, our construct was mainly accumulated in progenitor cells (Fig 4B–E) and preferentially enriched within EBs (Su(H)‐LacZ‐positive cells; compare Fig 4C with E). Expectedly, after inducing tissue damage, the restricted pattern was lost and the Dronc‐GFP‐myc was widely accumulated in all of the intestinal cell types (Appendix Fig S3D). Considering these findings, we combined the DBS‐S‐QF sensor with the EB marker Su(H)‐LacZ. This experiment showed the prevalent activation of DBS‐QF in EBs (Fig 4F and Appendix Fig S3E). Together, these findings correlated the functions of Dronc in progenitor cells with its specific accumulation and transient activation.
Figure 4. Dronc is preferentially accumulated and activated in progenitor cells.
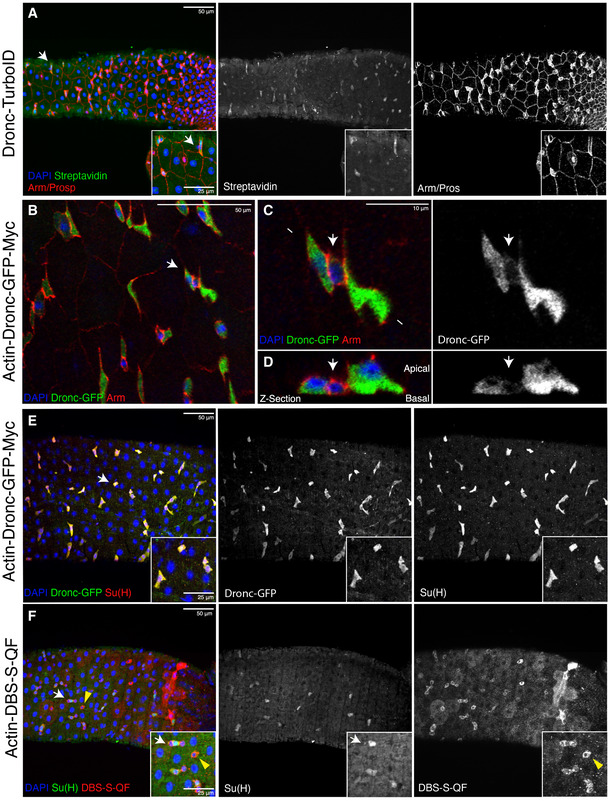
- Representative image of a Drosophila posterior midgut showing the expression of Armadillo (red membranes), Prospero (red nuclei of EEs) and Dronc‐TurboID (green shows Streptavidin‐labelled proteins by Dronc‐TurboID); note the preferential enrichment of Streptavidin staining is preferentially enriched in the intestinal progenitor cells (white arrows indicate an intestinal progenitor cell upregulating Dronc). Genotype: Dronc‐TurboID‐V5 (Masayuki Miura)/Tm6B.
- Representative image of a Drosophila posterior midgut showing the expression of Armadillo (red membranes) and Actin‐Dronc‐GFP‐Myc (green, anti‐GFP); note the preferential enrichment of Dronc expression in the small groups of cells formed by intestinal progenitor cells (arrow). Genotype: w 1118; Actin‐Dronc‐GFP‐Myc (attP‐VK37)/Cyo.
- High magnification of the picture shown in (B). The white arrow indicates a presumptive ISC showing low levels of Dronc compared with the flanking GFP + EBs. Genotype: w 1118; Actin‐Dronc‐GFP‐Myc (attP‐VK37)/Cyo. The arrow indicates a presumptive small ISC expressing high levels of Arm and low levels of Dronc‐GFP‐Myc.
- Transversal Z‐section of the cells shown in C. Genotype: w 1118; Actin‐Dronc‐GFP‐Myc (attP‐VK37)/Cyo. The arrow indicates a presumptive small ISC expressing high levels of Arm and low levels of Dronc‐GFP‐Myc.
- The enteroblast marker Su(H) (red, immunostaining against Beta‐galactosidase) strongly co‐localises with high levels of Dronc expression (green, immunostaining against GFP); white arrow indicates the enlarged area depicted in the insets. Genotype: w 1118, Su(H)GBE‐LacZ (Irene Miguel Aliaga); Actin‐Dronc‐GFP‐Myc (attP‐VK37)/Cyo.
- There is extensive overlap between the expression of the EB marker Su(H) (green, immunostaining against Beta‐galactosidase; white arrow) and the apical caspase reporter DBS‐S‐QF (red, immunostaining against HA), but it is also present in progenitor cells without Su(H) expression (yellow arrowheads). DAPI (Blue) labels cell nuclei in all the panels.
Data information: All of the experiments described in the figure apart from (F) were performed in Oxford medium following an experimental regime that protects the epithelial integrity at 29°C. The experiment shown in (F) was conducted at 25°C. Genotype: Females w 1118 Su(H)GBE‐LacZ/y1 w1118 UAS‐mCD8::GFP.L QUAS‐mtdTomato‐3xHA Act‐DBS‐S‐QF.
Dronc activation limits Notch signalling
Moderate‐high levels of Notch signalling facilitate the entry of intestinal progenitor cells into several differentiation programmes (Hakim et al, 2010; Kapuria et al, 2012; Zhai et al, 2017). Since Dronc deficiency facilitates the conversion of EBs into ECs, we assessed the genetic interplay between Notch signalling and Dronc. To this end, we simultaneously eliminated the expression of Dronc and Notch in progenitor cells using the esg‐Gal4 driver. This genetic manipulation induced phenotypes comparable to that of Notch deficiency alone, and therefore, the increase in cell size and EC features linked to Dronc deficiency were suppressed (compare Fig 5A–C with Figs 2B–E and EV3), even though we did notice a modest increase in the number of progenitor cells (Fig 5C). Conversely, the ectopic activation of the Notch pathway (Nintra) in Dronc‐mutant progenitor cells caused the rapid elimination of intestinal precursors from the epithelia (Fig 5D and E). Furthermore, the reduction of intestinal precursor was more pronounced than when Nintra was expressed alone (Fig 5F). In parallel to these genetic interactions, we observed that the transcriptional Notch‐signalling reporter, Su(H)‐LacZ, remained robustly upregulated within differentiated ECs mutant for Dronc, whilst paraquat treatment failed to replicate these results (compare Fig 5G–I). These data genetically located the function of Dronc upstream of the Notch pathway, likely acting as a negative regulator.
Figure 5. Dronc activation limits Notch signalling in the intestine.
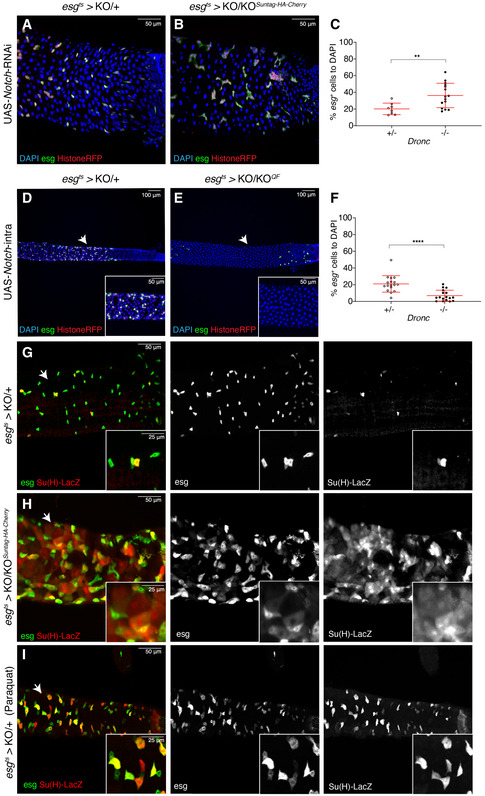
- Representative ReDDM labelling of a Drosophila Dronc heterozygous (+/−) intestine overexpressing an RNAi against Notch for 3 days; note the lack of fully differentiated Histone‐RFP cells as EC (red) without esg expression (green, GFP). DAPI staining labels cell nuclei in panels A, B, D and E. All of the experiments described in the figure were performed in Oxford medium following an experimental regime that protects the epithelial integrity. Genotype: w 1118 UAS‐Notch‐RNAi (Joaquin Navascués); esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/+.
- Representative ReDDM labelling of a Drosophila Dronc mutant homozygous (−/−) intestine overexpressing an RNAi against Notch for 3 post‐temperature shift at 29°C; note the lack of differentiated Histone‐RFP cells as EC (red) without esg expression (green, GFP), as well as the increase of GFP‐positive cells (compare A to B). Genotype: w 1118 UAS‐Notch‐RNAi (Joaquin Navascués); esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Quantification of the number of Histone‐RFP cells normalised to DAPI (proxy of progenitor cell proliferation obtained from the experiments shown in A and B panels); note the statically significant increase in Histone‐RFP‐positive cells in Dronc homozygous mutant intestines compared with controls (**P = 0.0080) (Quantifications were made using N ≥ 2 biological replicates; unpaired two‐tailed t‐test, +/− n = 8, −/− n = 15). Error bars represent standard deviation of the mean. Genotypes: +/−: w 1118 UAS‐Notch‐RNAi; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/+; −/−: w 1118 UAS‐Notch‐RNAi (Joaquin Navascués); esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Drosophila Dronc heterozygous intestine overexpressing the Notch intracellular domain for 7 days post‐temperature shift at 29°C; intestinal esg‐positive progenitor cells (green (GFP) and red (Histone‐RFP). Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/UAS‐Notchintra (Joaquin Navascués); TubG80ts UAS‐Histone‐RFP Dronc KO/+.
- Drosophila Dronc homozygous intestine overexpressing the Notch intracellular domain for 7d post‐temperature shift at 29°C; notice that the Dronc deficiency accelerates the elimination of intestinal progenitor cells induced by Notch overactivation (compare D and E). The white arrows indicate the position of insets 500 μm from the posterior region. Note the complete loss of esg‐labelled cells in this region. Genotype: w 1118; esg‐Gal4 UAS‐CD8‐GFP/ UAS‐Notchintra; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Relative number of esg‐positive cells to DAPI in either heterozygous or homozygous Dronc‐mutant esg cells overexpressing Notch intra; note the significant reduction of esg‐expressing cells (****P < 0.0001) (Quantifications were made using N ≥ 2 biological replicates; Mann–Whitney test, +/− n = 17, −/− n = 17). Error bars represent standard deviation of the mean. Genotypes: +/−: w 1118; esg‐Gal4 UAS‐CD8‐GFP/UAS‐Notchintra; TubG80ts UAS‐Histone‐RFP Dronc KO/+; −/−: w 1118; esg‐Gal4 UAS‐CD8‐GFP/UAS‐Notchintra; TubG80ts UAS‐Histone‐RFP Dronc KO/ UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Representative image of a Drosophila Dronc heterozygous intestines 7 days after transfer to 29°C. esg expression in green (GFP) labels all intestinal progenitors cells, whilst Su(H)‐lacZ (red) distinguishes a subpopulation of EBs within the esg‐expressing cells. Genotype: w 1118, Su(H)GBE‐LacZ; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/+.
- Representative image of a Drosophila Dronc KO intestine 7 days after transfer to 29°C. Note, the transcription of Su(H) in large cell GFP (−), suggestive of an aberrant upregulation of Notch signalling within fully differentiated ECs. Genotype: w 1118, Su(H)GBE‐LacZ; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
- Representative image of a 7d Drosophila Dronc heterozygous intestines following a 16‐h treatment with paraquat. Note the absence of large Su(H) positive, GFP (−) cells compared with (H). The white arrows indicate the enlarged area depicted in the insets. Genotypes: +/−: w 1118, Su(H)GBE‐LacZ; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/+; −/−: w 1118, Su(H)GBE‐LacZ; esg‐Gal4 UAS‐CD8‐GFP/+; TubG80ts UAS‐Histone‐RFP Dronc KO/UAS‐Flippase (BL8209) FRT Dronc‐GFP‐APEX FRT suntag‐HA‐Cherry.
Data information: (D–H) The white arrows indicate the enlarged area depicted in the insets.
Discussion
Non‐apoptotic Dronc activation contributes to ensuring gut epithelial homeostasis
Our caspase lineage‐tracing experiments (Baena‐Lopez et al, 2018a) and genetic manipulations have suggested a non‐apoptotic role for caspase activation in the intestinal progenitor cells, under non‐regenerative conditions. Supporting this conclusion, the lack of essential apoptotic regulatory components (e.g. the terminal effector caspases), failed to alter the intestinal epithelial features under our experimental regime (Fig EV4). On the contrary, Dronc activation limits the number of progenitor cells, and their unintended conversion from EBs into ECs (Figs 2 and EV3). These findings support the hypothesis that Dronc contributes to the preservation of intestinal progenitor cells in quiescence state through mechanisms independent of apoptosis. Importantly, caspase‐9 deficiency in human intestinal precursors results in excessive proliferation and poor differentiation (Xu et al, 2013). Therefore, our findings could be relevant to understanding the origin of human intestinal malignancies. Beyond the implications in the intestinal system, our results confirm the non‐apoptotic and caspase‐dependent regulation of stem cell function (Aram et al, 2017; Bell & Megeney, 2017; Baena‐Lopez et al, 2018b).
Caspases can modulate the intestinal epithelial properties through non‐apoptotic and apoptotic molecular mechanisms
Several reports have shown the ability of caspases to modulate the proliferation and differentiation of intestinal precursors in regenerative conditions (Amcheslavsky et al, 2009; Jin et al, 2013; Jin et al, 2017). In particular, the cleavage of the chromatin regulator Brahma mediated by the effector caspases restrains the proliferation and differentiation of ISCs after tissue damage (Jin et al, 2013). Here, we show that Dronc is required to prevent the increase in number of intestinal precursor cells as well as their unintended differentiation in non‐regenerative conditions. These roles of Dronc are unlikely to be correlated with expression of Brahma, since the effector caspases are largely dispensable in our experimental setting (Fig EV4). Alternatively, they appear to be linked to the Dronc‐dependent modulation of Notch signalling (Fig 5). This conclusion is supported by the upregulation of Notch target genes (e.g. Su(H)) in Dronc‐mutant conditions and classical genetic interaction experiments (Fig 5). A physical interaction between Dronc and the Notch pathway inhibitor referred to as Numb was previously reported in the Drosophila nervous system (Ouyang et al, 2011); however, the genetic manipulation of this factor in our cellular scenario did not cause noticeable phenotypes (Appendix Fig S3F and G). This was expected to some extent since numb only participates in the conversion of ISCs into EEs (Salle et al, 2017). Future studies focused on investigating whether Dronc is able to alter Notch protein features (e.g. stability, internalisation…) or the expression of alternative Notch target genes (e.g. members of the Enhancer of Split family) could better define the interplay between this signalling pathway and Dronc. Beyond the novel non‐apoptotic functions, caspases can also limit the number of progenitor cells generated in response to different stimuli via the apoptosis programme (Reiff et al, 2019), thus preventing gut hyperplasia in regenerative conditions. Together, these data illustrate the diversity of apoptotic and non‐apoptotic caspase‐dependent mechanisms ensuring intestinal homeostasis.
Dronc activation helps to preserve the intestinal progenitor cells in quiescence
Our experiments have revealed that the elimination of Dronc expression in each type of intestinal progenitor cell do not cause major alterations in the intestinal epithelium (Fig 3H). However, the simultaneous elimination of Dronc in both progenitor cells (ISC and EBs) robustly increases their number and induces the conversion of EBs into ECs (Fig 2). These data suggest that both types of progenitor cells require Dronc activation but having it in one of them suffices to maintain the intestinal epithelium in quiescence; therefore, Dronc could modulate the coordinated activity of progenitor cells. Our data also indicate that the functional requirement of Dronc is not equivalent in both progenitor cells and is highly correlated with its level of expression in each type of progenitor cell (Fig 4). From a molecular perspective, the coordinated behaviour of progenitor cells has been correlated with genetic factors expressed in both cell types [e.g. Sox protein family (Meng & Biteau, 2015; Zhai et al, 2015; Chen et al, 2016; Zhai et al, 2017; Meng et al, 2020)]. The upregulation of Sox proteins initiates signalling feedback loops that reactivate intestinal precursors, and EC differentiation in either homeostatic or stress conditions (Meng & Biteau, 2015; Zhai et al, 2015; Chen et al, 2016; Zhai et al, 2017). Furthermore, Sox expression can increase the activation levels of Notch signalling through the upregulation of Delta in ISCs, whilst facilitating the conversion of EBs into ECs (Zhai et al, 2017). Exploring the interplay between caspases and these factors could provide novel insights into the molecular implementation of caspase functions in the future. Beyond the Sox protein family, caspases could modulate cell–cell interactions between progenitor cells (Suzanne & Steller, 2009), the cytoskeleton dynamics and trafficking events (Duclos et al, 2017), thus influencing the activation levels of numerous signalling routes, including Notch (Zhai et al, 2017). Finally, Dronc could be buffering signalling fluctuations in different pathways (e.g. Notch) triggered by diverse intracellular, developmental or environmental stimuli (Khalil et al, 2014; Bell & Megeney, 2017; Weaver et al, 2020) (e.g. toxic agents, dietary conditions, infection). At this point, the experimental validation of these and other hypotheses is beyond reach of this manuscript; however, we can conclude that non‐apoptotic Dronc activation modulates Notch signalling and the coordination of intestinal progenitor cells. Importantly, although Dronc appears to prevent the proliferation and differentiation of progenitor cells, its deficiency is not sufficient to reactivate these cells since not all of the Dronc mutant intestines displayed phenotypic manifestations. Therefore, we propose that caspases could be buffering the effect of other primary inducers promoting the exit from quiescence (e.g. environmental stress or intracellular signalling imbalance). Supporting this hypothesis, it has been shown that non‐apoptotic caspase activation can ensure intracellular protein homeostasis (proteostasis) (Bell et al, 2016; Bell & Megeney, 2017) and can counteract the upregulation of signalling stress pathways (Fernando et al, 2002; Khalil et al, 2014; Bell et al, 2016; Weaver et al, 2020).
Tumour suppressor roles of caspases independent of apoptosis
Apoptosis is the main tumour suppressor mechanism linked to the caspases (Hanahan & Weinberg, 2011), and conceivably, sustained caspase activation can induce the elimination of intestinal progenitor cells (Reiff et al, 2019). However, caspases in the intestinal system display tumour suppressor activities independent of apoptosis. Along these lines, they can limit the proliferation and differentiation of progenitor cells (Jin et al, 2013; Lee et al, 2015) and our findings suggest that they help to preserve the progenitor cells in quiescence. Collectively, these data support the hypothesis that caspases can act as tumour suppressor genes through non‐apoptotic mechanisms.
Materials and Methods
Fly husbandry
All fly strains used are described at https://flybase.org/ unless otherwise indicated. Primary Drosophila strains and crosses were routinely maintained on Oxford fly food. The fly food has three basic components that should be properly mixed (food base, Nipagin mix and Acid mix). The Exact amount of the three components are described next.
Base composition per litre of fly food: agar (3 g/l, Fisher Scientific UK Ltd, BP2641‐1), malt (64.3 g/l), molasses (18.8 g/l), maize (64.3 g/l), yeast (13 g/l), soya (7.8 g/l), water (1 l).
Nipagin mix per litre of fly food: Methyl‐4‐hydroxybenzoate (nipagin) (2.68 g, scientific labs, cat. W271004‐10KG‐K), absolute ethanol (25.1 ml, Fisher), water (1.3 ml).
Acid mix per litre of fly food: 5% phosphoric acid (Phosphoric Acid 85% Insect Cell Culture, cat. P5811‐500G) in Propionic acid (5 ml, Propionic Acid Free Acid Insect Cell Culture, cat. P5561‐1L).
Live yeast was added to each tube before transferring adult flies unless specified otherwise. Specific experiments also used Drosophila Quick Mix Medium Food (Blue) obtained from Blades Biological Ltd (DTS 070). 1 g of Drosophila Quick Mix Medium (Blue) was mixed with 3 ml of ddH2O in these experiments.
Conditional knockout and experimental regime during adulthood
Females were crossed with males at 18°C to prevent developmental lethality and transgene expression before adulthood. Adult female flies were then left mating with their siblings during 48 h and finally transferred to 29°C. At 29°C, the Gal80 repression of Gal4 is not effective allowing transgene expression. Until the dissection point, experimental specimens were transferred every 2 days to vials with fresh food containing yeast, or without yeast when using the Drosophila Quick Mix Media (Appendix Fig S1A).
Paraquat treatment
The Drosophila were dry starved for 4 h. Subsequently, the flies were transferred to an empty fly vial in which the fly food was replaced by a flug soaked in a solution of 5% sucrose and 6.5 mM Paraquat. Flies were left in this vial for 16 h prior dissection.
Molecular cloning and plasmid generation details
All PCRs were performed with Q5 High‐Fidelity polymerase from New England Biolabs (NEB, M0492L). Standard subcloning protocols and HiFi DNA Assembly Cloning Kit (NEB, E5520S) were used to generate all the DNA plasmids. Transgenic lines expressing the new Dronc rescue constructs were obtained by attP/attB PhiC31‐mediated integration. To this end, all the DNA plasmids were injected in Drosophila embryos containing the Dronc KO‐reintegration site using Bestgene Inc. Fly strains generated will be deposited at the Bloomington Stock Centre. Whilst resource transfer is completed, reagents will be provided upon request. Description of RIV‐Gal4 and plasmid backbones used for generating the different DNA rescue constructs can be found in Baena‐Lopez et al (2013).
pTV‐Cherry‐DroncKO. Dronc‐targeting vector
We first amplified by PCR the homology arms for generating the gene targeting of Dronc. Genomic DNA extracted through standard protocols from w 1118 flies was used as a template. The sequences of the primers used are as follow:
5ʹ homology arm Forward primer with NotI restriction site: 5ʹ attatGCGGCCGCAAGTTGATGCAGCCTTCTGC 3ʹ
5ʹ homology arm Reverse primer with KpnI restriction site: 5ʹ aattatGGTACCTCCGGTGACTCCGCTTATTGG 3ʹ
3ʹ homology arm Forward primer with bglII restriction site: 5ʹ attgccaAGATCTACTGGACATTTTATCATTCC 3ʹ
3ʹ homology arm Reverse primer with AvrII restriction site: 5ʹ attatggCCTAGGTCAAATCTGTTAATTTACG 3ʹ
We then subcloned the PCR products into the targeting vector pTV‐Cherry. The 5ʹ and 3ʹ homology arms were cloned into pTV‐Cherry as a NotI‐KpnI and BglII‐AvrII fragments, respectively. The DroncKO behaves as previously described null alleles of the gene and is homozygous lethal during pupal development. The molecular validation of the allele was also made by PCR. First, we extracted the DNA from 10 larvae using the Quick genomic DNA prep protocol described in the link below. http://francois.schweisguth.free.fr/protocols/Quick_Fly_Genomic_DNA_prep.pdf.
The sequence of the primers used for performing the PCR were:
Forward KO validation primer: 5ʹ TGAGCAGCTGTTGGCATTAGG 3ʹ
Reverse KO validation primer: 5ʹ AGAGAATACCAATCATACTGC 3ʹ
The PCR conditions were 30 s at 64°C of annealing temperature and 1 min at 72°C of extension. The PCRs were made using the Q5 High‐Fidelity polymerase from New England Biolabs (NEB, M0492L).
RIV‐DroncKO‐Gal4
RIV‐Gal4 plasmid was injected in the DroncKO‐reintegration site for generating the DroncKO ‐Gal4 line.
RIV‐DroncKO‐Dronc WT‐suntag‐HA
Two fragments were amplified by PCR using the full‐length wild‐type cDNA of Dronc as a template (LP09975 cDNA clone from Drosophila Genomics Resource Centre). This cDNA also contained the flanking untranslated regions of Dronc in 5ʹ and 3ʹ. We also appended a suntag‐HA‐tag to the C‐terminal end of the Dronc open reading frame, before the stop codon. The PCR fragments were then subcloned using HiFi DNA Assembly Cloning Kit into a PUC57 plasmid backbone previously opened with NotI‐KpnI. The whole fragment was then transferred into the RIV [MCS‐white (Baena‐Lopez et al, 2013)] as a NotI‐KpnI fragment. The plasmid was finally injected in the Dronc KO‐reintegration site. The primer sequences used for generating the rescue plasmid are next indicated.
Forward primer 1: 5ʹ tctagAGGGGGCTACCCATACGACGTCCCTGACTATGCGTAAgctagcttgccgccactggacattttatcattccgg 3ʹ
Forward primer 2: 5ʹ gacggccagtgcggccgcagatctcctaggccggaacgcgtggaagccatatccggaatgcagccgccggagctcgagattgg 3ʹ
Reverse primer 1: 5ʹ tctagAGGGGGCTACCCATACGACGTCCCTGACTATGCGTAAgctagcttgccgccactggacattttatcattccgg 3ʹ
Reverse primer 2: 5ʹ caaaaattagactctttggccttagtcgggtaccggcgcgccactcgagcactagtTCTGGCTGTGTatatactgg 3ʹ
Importantly, flies expressing this construct are viable, fertile and morphologically normal. Only the adult wings are less transparent than normal and sometimes are not expanded normally.
RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT QF. Conditional Dronc allele followed by QF
We first modified the WT cDNA of Dronc adding a GFP‐Apex2 chimeric fragment. This fragment was appended in frame before the stop codon of Dronc in order to facilitate biochemical approaches not used in this manuscript. The cDNA of Dronc and the GFP‐Apex2 chimeric fragments were amplified by PCR from the plasmids droncKO‐Dronc‐WT and pCDNA‐conexing‐EGFP‐APEX2 (addgene #49385), respectively. The sequences of the primers used to that end were:
Forward primer 1: 5ʹ tccggagcggccgcagatctCCGGAACGCGTGGAAGCCATATCCG 3ʹ
Forward primer 2: 5ʹ TCCCGGGTTTTTCAACGAAggcggaatggtgagcaagggcgaggagc 3ʹ
Reverse primer 1: 5ʹ tcgcccttgctcaccattccgccTTCGTTGAAAAACCCGGGATTG 3ʹ
Reverse primer 2: 5ʹ atgtccagtggcggcaagctagcttaggcatcagcaaacccaagc 3ʹ
The PCR fragments were then subcloned using HiFi DNA Assembly Cloning Kit into RIV‐DroncKO‐Dronc‐WT‐suntag‐HA previously opened with BglII‐NheI. The entire construct was finally transferred into RIV [FRT‐MCS2‐FRT QF; pax‐Cherry (Baena‐Lopez et al, 2013)] as a NotI‐SpeI fragment. Notice that the extra sequences appended to Dronc (the FRT sites and the QF fragment) do not compromise the rescue ability of the construct. Indeed, these flies are identical to RIV‐DroncKO‐Dronc‐WT‐suntag‐HA. Homozygous flies expressing QF upon FRT‐rescue cassette excision die during metamorphosis indicating this allele in such configuration behaves as previously described null alleles.
RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT suntag‐HA‐Cherry. Conditional Dronc allele suntag‐HA‐Cherry
We subcloned a newly designed PCR product suntag‐HA‐Cherry in PUC57‐DroncKO‐Dronc WT‐suntag‐HA by using HiFi DNA Assembly Cloning Kit. The PUC57‐DroncKO‐Dronc WT‐suntag‐HA backbone vector was opened with AvrII‐NsiI. The primers used for generating the suntag‐HA‐Cherry peptide were:
Forward primer 1: 5ʹgccagtgcggccGCagatctCCTAGGcccgggtttttcaacgaagggggcgaggagttgctgaGCAAAAATTATCATTTGGAGAacgaagtagcacgactaaag 3ʹ
Forward primer 2: 5ʹgtagcacgactaaagaaagggtccggatcgggttctagagggggctacccatacgacgtccctgactatgcgGGGaattCCAACatggtgagcaagggcg 3ʹ
Reverse primer 1: 5ʹcgcccttgctcaccatGTTGGaattCCCcgcatagtcagggacgtcgtatgggtagccccctctagaacccgatccggaccctttctttagtcgtgctac 3ʹ
Reverse primer 2: 5ʹggacgagctgtacaagtaagacgtcgtcgaGGGTACCTctagcttgccgccactggacattttatcattccggatgcatttttaaccgcatttatgt 3ʹ
Then, the suntag‐HA‐Cherry fragment was extracted from the new PUC57‐DroncKO‐Dronc‐WT‐suntag‐HA‐Cherry vector a AvrII‐blunted ‐ClaI and transferred to RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT QF previously opened with AvrII‐blunted ‐ClaI. Homozygous flies expressing suntag‐HA‐Cherry peptide under the physiological regulation of Dronc die during metamorphosis indicating this allele behaves as previously described null alleles.
RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT Dronc‐FLCA‐suntag‐HA‐Cherry and RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT Dronc‐FLCAEA‐suntag‐HA‐Cherry. Conditional Dronc alleles FLCA and FLCAEA‐suntag‐HA‐Cherry
We first generated two point mutations through gene synthesis (Genewiz) in the wild‐type cDNA of Dronc that caused the following amino acid substitutions, C318A and E352A. This version of Dronc is enzymatically inactive (C318A) and cannot be either processed during the proteolytic activation steps of Dronc (E352A). This fragment was subcloned in PUC57‐DroncKO‐Dronc‐WT‐suntag‐HA‐Cherry as a BglII‐XmaI fragment, thus replacing the wild‐type version of Dronc by the mutated. Finally, the DNA sequence was transferred to the RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT QF plasmid as an AvrII‐ClaI fragment. Homozygous flies expressing this mutant form of Dronc die during metamorphosis indicating this allele behaves as previously described null alleles. An equivalent cloning strategy was followed to generate the FLCA allele, although in that case a single mutation (C318A) was introduced in the Dronc wild‐type template through DNA synthesis.
RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT Dronc‐deltaCAEA‐suntag‐HA‐Cherry. Conditional Dronc allele deltaCAEA‐suntag‐HA‐Cherry
We generated a PCR product that deletes the CARD domain of Dronc using the following primers and as template for the PCR RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT Dronc‐FLCAEA‐suntag‐HA‐Cherry.
Forward primer 1: 5ʹ ggccagtgcggccGCCCTAGGGTTTaaacggggaatgggcaattGtctggatgcggcc 3ʹ
Reverse primer 1: 5ʹ catGTTGGaattccccgcatagtcagggacgtcgtatgggtagccccc 3ʹ
The PCR product was subcloned in PUC57‐DroncKO‐Dronc‐suntag‐HA‐Cherry as a NotI‐EcoRI fragment, thus inserting the truncated and catalytically inactive version of Dronc in frame with the Suntag‐HA‐Cherry peptide. Finally, the DNA sequence was transferred to the RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT QF plasmid as an AvrII‐PasI fragment. Homozygous flies expressing this mutant form of Dronc die during metamorphosis indicating this allele behaves as previously described null alleles.
RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT Dronc‐FLWT‐suntag‐HA‐Cherry. Conditional Dronc allele Dronc‐FLWT‐suntag‐HA‐Cherry
We generated a PCR product using as a template for the PCR RIV‐DroncKO‐Dronc WT‐suntag‐HA and using the following primers.
Forward primer 1: 5ʹ ggccagtgcggccgcagatctcctaggccggaacgcgtggaagccatatccggaatgcagccgccgga 3ʹ
Reverse primer 1: 5ʹ cccttgctcaccatGTTGGaattCCCcgcatagtcagggacgtcgtatggg 3ʹ
The PCR product was subcloned in PUC57‐DroncKO‐Dronc‐suntag‐HA‐Cherry as a NotI‐EcoRI fragment, thus inserting the wild‐type version of Dronc in frame with the suntag‐HA‐Cherry peptide. Finally, the DNA sequence was transferred to the RIV‐DroncKO FRT‐DroncWT‐GFP‐Apex‐FRT QF plasmid as an AvrII‐PasI fragment. Heterozygous flies expressing this mutant form rescue the pupal lethality associated with Dronc insufficiency.
Plasmid generation details of Actin‐Dronc‐CA‐GFP‐Myc
We first generated one point mutation through gene synthesis (Genewiz) in the wild‐type cDNA of Dronc that causes the following amino acid substitution C318A. This version of Dronc is enzymatically inactive. Also, we appended to the C terminus a suntag and a HA peptide sequence in frame with the open reading frame (ORF) of Dronc. Downstream of the ORF was included 3ʹUTR of Dronc present in the genomic locus. Extra restriction sites were added at the 5ʹ and 3ʹ of the construct to facilitate future subcloning projects. The entire construct was subcloned in PUC57 as a Not‐KpnI fragment. We then opened this vector with SmaI and NheI; this enzymatic digestion eliminates the C‐terminal tagging of Dronc (suntag‐HA) whilst retaining the 3ʹUTR. Using HiFi DNA assembly, we inserted a PCR product that encodes for a modified version of GFP with a Myc tag appended at the C‐terminal end. The primers used to amplify the modified GFP‐Myc were:
Forward primer 1: 5ʹ GCTTTAATAAGAAACTCTACTTCAATcccgggtttttcaacgaagggggcATGATCAAGATCGCCACCAGGAAGTACC 3ʹ
Forward primer 2: 5ʹ CGTGACCGCCGCCGGGATCACGGAAACCGATGGCGAGCTGTTCACCGGGGTGG 3ʹ
Reverse primer 1: 5ʹ CCACCCCGGTGAACAGCTCGCCATCGGTTTCCGTGATCCCGGCGGCGGTCACG 3ʹ
Reverse primer 2: 5ʹ gataaaatgtccagtggcggcaagctagCttacaggtcctcctcgctgatcagcttctgctcGTTAGGCAGGTTGTCCACCCTCATCAGG 3ʹ
The template used to obtain the GFP‐Myc PCR product was extracted from genomic DNA of flies containing the construct UAS‐GC3Ai (Schott et al, 2017). The construct was finally subcloned as a NotI‐XhoI fragment in an Actin‐polyA vector of the lab previously opened with NotI‐PspXI. Sequence of the plasmid will be provided upon request until the vector is deposited in a public repository.
Immunohistochemistry
Adult mated female Drosophila Intestines were dissected in ice‐cold PBS. Following dissection, the intestines were immersed for 6 s in wash solution (0.7% NaCl, 0.05% Triton X‐100) heated to approximately 90°C. Subsequently, the intestines were rapidly cooled in ice‐cold wash solution. The intestines were then rapidly washed in PBT (0.3%) before blocking for at least 1 h in 1% BSA‐PBT (0.3%). Primary antibodies were incubated overnight at 4°C and secondary antibodies at room temperature for 2 h, diluted in blocking solution. Primary antibodies used were Goat Anti‐GFP (1:200, Abcam, ab6673) Chicken anti‐Beta‐galactosidase (1:200, Abcam, Ab9361); Rabbit Anti‐HA (1:1,000, Cell Signalling, C29F4); Rabbit anti‐Pdm1 (1:2,000, kind gift from Yu Cai), Mouse Anti‐Armadillo (1:50, DSHB, N2 7A1 ARMADILLO‐c); Mouse Anti‐Prospero (1:20; DSHB, MR1A) and Rabbit Anti‐P35 (1:100, Novus Biologicals, NB100‐56153). The secondary antibodies used were DAPI (1:1,000, Thermo Scientific, 62248); Goat Alexa 488 anti‐Chicken (1:200, Life technologies, A11039); Donkey Alexa‐488 anti‐Goat (1:200, Life Technologies, A1105); Donkey Alexa‐555 anti‐Rabbit (1:200, Life Technologies, A31572),Donkey Alexa‐647 anti‐Rabbit (1:200, Life Technologies, A31573); Donkey Alexa‐555 Anti‐Mouse (1:200, Life Technologies, A31570) and Donkey Alexa‐647 Anti‐chicken (1:200, Jackson, 703‐605‐155).
EdU assay
Adult mated female Drosophila Intestines were dissected in ice‐cold PBS. Following dissection, the intestines were transferred to an Eppendorf tube and incubated with 20 µM EDU (Click‐iT™ EdU Cell Proliferation Kit for Imaging, Alexa Fluor™ 647 dye, C10340) in PBS for 60 min at room temperature. Following treatment, intestines were rapidly washed in PBS, before fixation in 4% paraformaldehyde (Thermo Fisher Scientific, 433689L) diluted in PBS for 1 h. The intestines were then washed before incubation with the Click‐iT™ reaction cocktail according to the manufacturer’s instructions. In order to avoid unspecific signal, the intestines were incubated with the blocking solution (1% BSA‐PBT (0.3%)) at 4°C for at least 1 h. Primary antibodies were incubated at 2× concentration at 4°C overnight. Standard protocols were followed to complete the staining with secondary antibodies.
Immunoprecipitation and Western blot
Twenty flies of each genotype were snap‐frozen at −80°C, thawed, macerated on ice in 200 μl of RIPA buffer (complemented with protease inhibitor), cleared at 20k g for 20 min and supernatant toped‐up with PbS + 0.3% Triton X‐100 to 500 μl. 50 μl of each sample was set aside, and the remaining 450 μl incubated with 15 μl of HA magnetic beads (Pierce) pre‐washed three times in PbS + 0.3% Triton X‐100 head‐over‐head at 4°C on a spinning wheel for 90 min. Beads were washed three times in PbS + 0.3% Triton X‐100. Bound material was eluded from the beads in 50 μl Laemmli buffer and the equivalent of the lysate of five flies loaded on a 4–12% gradient gel. Western blot was revealed with rabbit HA antibody (CST 1:1,000) or beta‐actin (DSHB 1:500).
Generation of genetic mosaics
Females yw hs‐Flp1.22; Sp/Cyo; FRT80A UbiGFP/TM6B were either crossed with males w1118 ‐; Dronc I29 FRT80/TM6b (Experimental) or w1118; +/+; FRT80 ry +/FRT80 ry + (Control). Flies were selected every 2 days and then heat shocked for 90 min at 37°C. Following the heat shock treatment, flies were returned to 25°C for 7 days until dissection.
Imaging of fixed samples
Fluorescent imaging of the R5 posterior region (Dutta et al, 2015) of Drosophila intestines were performed using the Olympus Fluoview FV1200 and associated software. Z‐stacks were taken using either the 40× or 10× lenses. Acquired images were processed and quantified using automated Fiji/ImageJ (Schindelin et al, 2012; Rueden et al, 2017) macros or manually when automatisation was not possible. Generally, Z‐stacks were projected, and the channels split. The “despeckle” tool was utilised to remove noise. The image was then “thesholded” and the “watershed” tool used to segment joined cells. To count the number of objects, the “Analyse Particles…” function was utilised in automated quantification, and for manual counting, “cell counter”. Figures were produced with Adobe Illustrator CC 2017.
Statistical analysis
Microsoft Excel was used to complete the basic numerical preparation of the data. The data were subsequently collated in GraphPad Prism (8). The “identity outlier” analysis was utilised using the ROUT method with a Q value of 1% (All N numbers listed in figures are prior to this analysis). The cleaned data were then tested for normality using the D’Agostino‐Pearson omnibus normality test except for qPCR data which were tested using the Shapiro–Wilk normality test. All subsequent analysis is referenced in Figure Legends. The P value format used is as follows: ns = P> 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
RNA extraction and cDNA synthesis of Drosophila intestines and qPCR
Around 20 adult mated female Drosophila intestines were dissected in ice‐cold PBS. Following dissection, the intestines were transferred to autoclaved Eppendorf tubes containing 350 μl of RLT Buffer plus from the RNeasy Plus Micro Kit (QIAGEN Cat. No 74034) with 1% v/v of 2‐mercaptoethanol (Sigma‐Aldrich M6250). The intestines were homogenised using a new 1.5 ml pestle (Kimble 749521‐1590) for each sample. The RNA was then extracted using the protocol and materials outlined in the RNeasy Plus Micro Kit. RNA quantity in samples was assessed twice using the Nanodrop Lite Spectrophotometer (Thermo Fisher Scientific) and the average determined. 500 μg of RNA was synthesised to cDNA (Thermo Fisher Scientific Maxima First‐Strand cDNA Synthesis Kit—K1671). QPCR was performed using reagents and protocols from QIAGEN QuantiTect SYBR® Green PCR Kit (Cat No./ID: 204145) and using the QIAGEN Rotor‐Gene Q.
The primer sequences were:
Rpl32 (Gomez‐Lamarca et al, 2018):
Forward: 5ʹ ATGCTAAGCTGTCGCACAAATG 3ʹ
Reverse: 5ʹ GTTCGATCCGTAACCGATGT 3ʹ
Alpha‐Trypsin* (PD44223):
Forward: 5ʹ ATGGTCAACGACATCGCTGT 3ʹ
Reverse: 5ʹ CTGGCTCTGGCTAACGATGT 3ʹ
Amylase‐D* (PD40005):
Forward: 5ʹ GCATAGTGTGCCTCTCCCTC 3ʹ
Reverse: 5ʹ TACGACCGGATGCGTAGTTG 3ʹ
Jon65Aiii (Bozler et al, 2017):
Forward: 5ʹ AACACCTGGGTTCTCACTGC 3ʹ
Reverse: 5ʹ TCAGGGAAATGTCGTTCCTC 3ʹ
*Primers were sourced from the QPCR FlyPrimerBank tool (Hu et al, 2013).
Author contributions
Conceptualisation: LAB‐L; Plasmid generation, molecular biology protocols, allele preparation: DAN, JA, LAB‐L; Experimental design, discussion: LA, LAB‐L; Experimental work: LA; Immunoprecipitation, Western blot: FW; Writing manuscript, correction: LA, LAB‐L. Figures: LA, LAB‐L; Comments, approval: all co‐authors.
Conflict of interest
All authors declare that they have no conflicts of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
The authors would like to thank Irene Miguel‐Aliaga, María Dominguez, Andreas Bergmann, Joaquín Navascués and Iswar Hariharan for sharing various flies and reagents; Yu CAI (Temasek Life Sciences Laboratory Limited) for generously sharing the Pdm1 antibody; Joaquín Navascués for generously sharing reagents and protocols; Antonello Zeus for his intellectual input at the initial stages of the project; and the Caspase Lab members for their critical reading of the manuscript and invaluable suggestions. This work has been supported by Cancer Research UK 560C49979/A17516 and the John Fell Fund from the University of Oxford 162/001. L.A.Baena‐Lopez is a CRUK Career Development Fellow (C49979/A17516) and an Oriel College Hayward Fellow. J. Alonso was a CRUK postdoctoral scientist associated with the grant code previously described. F. Wendler is a CRUK postdoctoral scientist associated with the grant code previously described. D. Antoni. Nahotko was a summer student supported by the ERASMUS (+) programme. L. Arthurton was a PhD student supported by the Edward Penley Abraham Research Fund.
EMBO Reports (2020) 21: e48892.
Data availability
All the raw images and data sets use for quantification purposes in the manuscript will be distributed upon reasonable request. The new fly strains generated for this manuscript will be deposited in the Bloomington Drosophila Stock Center (https://bdsc.indiana.edu/) and distributed upon request whilst the deposit is processed. This study includes no data deposited in external repositories.
References
- Ali S, Jain SK, Abdulla M, Athar M (1996) Paraquat induced DNA damage by reactive oxygen species. Biochem Mol Biol Int 39: 63–67 [DOI] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT (2009) Tissue damage‐induced intestinal stem cell division in Drosophila . Cell Stem Cell 4: 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonello ZA, Reiff T, Ballesta‐Illan E, Dominguez M (2015) Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR‐8‐Escargot switch. EMBO J 34: 2025–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aram L, Yacobi‐Sharon K, Arama E (2017) CDPs: caspase‐dependent non‐lethal cellular processes. Cell Death Differ 24: 1307–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arama E, Baena‐Lopez LA, Fearnhead HO (2020) Non‐lethal message from the Holy Land: The first international conference on non‐apoptotic roles of apoptotic proteins. FEBS J 10.1111/febs.15547 [DOI] [PubMed] [Google Scholar]
- Baena‐Lopez LA, Alexandre C, Mitchell A, Pasakarnis L, Vincent JP (2013) Accelerated homologous recombination and subsequent genome modification in Drosophila . Development 140: 4818–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena‐Lopez LA, Arthurton L, Bischoff M, Vincent JP, Alexandre C, McGregor R (2018a) Novel initiator caspase reporters uncover previously unknown features of caspase‐activating cells. Development 145: dev170811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena‐Lopez LA, Arthurton L, Xu DC, Galasso A (2018b) Non‐apoptotic caspase regulation of stem cell properties. Semin Cell Dev Biol 82: 118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RA, Al‐Khalaf M, Megeney LA (2016) The beneficial role of proteolysis in skeletal muscle growth and stress adaptation. Skelet Muscle 6: 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RAV, Megeney LA (2017) Evolution of caspase‐mediated cell death and differentiation: twins separated at birth. Cell Death Differ 24: 1359–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozler J, Kacsoh BZ, Chen H, Theurkauf WE, Weng Z, Bosco G (2017) A systems level approach to temporal expression dynamics in Drosophila reveals clusters of long term memory genes. PLoS Genet 13: e1007054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xu N, Huang H, Cai T, Xi R (2016) A feedback amplification loop between stem cells and their progeny promotes tissue regeneration and tumorigenesis. Elife 5: e14330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TC, Akey IV, Yuan S, Yu Z, Ludtke SJ, Akey CW (2017) A near‐atomic structure of the dark apoptosome provides insight into assembly and activation. Structure 25: 40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SK, Akdemir F, Chen P, Lu WJ, Mills K, Daish T, Kumar S, Rodriguez A, Abrams JM (2004) The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila . Dev Cell 7: 897–907 [DOI] [PubMed] [Google Scholar]
- Christich A, Kauppila S, Chen P, Sogame N, Ho SI, Abrams JM (2002) The damage‐responsive Drosophila gene sickle encodes a novel IAP binding protein similar to but distinct from reaper, grim, and hid. Curr Biol 12: 137–140 [DOI] [PubMed] [Google Scholar]
- Crawford ED, Wells JA (2011) Caspase substrates and cellular remodeling. Annu Rev Biochem 80: 1055–1087 [DOI] [PubMed] [Google Scholar]
- Ding AX, Sun G, Argaw YG, Wong JO, Easwaran S, Montell DJ (2016) CasExpress reveals widespread and diverse patterns of cell survival of caspase‐3 activation during development in vivo . Elife 5: e10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Marshall OJ, Dayton H, Brand AH, Perrimon N (2018) Drosophila intestinal stem and progenitor cells are major sources and regulators of homeostatic niche signals. Proc Natl Acad Sci USA 115: 12218–12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos C, Lavoie C, Denault JB (2017) Caspases rule the intracellular trafficking cartel. FEBS J 284: 1394–1420 [DOI] [PubMed] [Google Scholar]
- Dutta D, Dobson AJ, Houtz PL, Glasser C, Revah J, Korzelius J, Patel PH, Edgar BA, Buchon N (2015) Regional cell‐specific transcriptome mapping reveals regulatory complexity in the adult Drosophila midgut. Cell Rep 12: 346–358 [DOI] [PubMed] [Google Scholar]
- Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA (2002) Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci USA 99: 11025–11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Lamarca MJ, Falo‐Sanjuan J, Stojnic R, Abdul Rehman S, Muresan L, Jones ML, Pillidge Z, Cerda‐Moya G, Yuan Z, Baloul S et al (2018) Activation of the notch signaling pathway in vivo elicits changes in CSL nuclear dynamics. Dev Cell 44: 611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal L, McCall K, Agapite J, Hartwieg E, Steller H (2000) Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J 19: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Ohlstein B (2015) Stem cell regulation. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science 350: aab0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim RS, Baldwin K, Smagghe G (2010) Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol 55: 593–608 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM (1994) Expression of baculovirus P35 prevents cell death in Drosophila . Development 120: 2121–2129 [DOI] [PubMed] [Google Scholar]
- Hu Y, Sopko R, Foos M, Kelley C, Flockhart I, Ammeux N, Wang X, Perkins L, Perrimon N, Mohr SE (2013) FlyPrimerBank: an online database for Drosophila melanogaster gene expression analysis and knockdown evaluation of RNAi reagents. G3 (Bethesda) 3: 1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Foe I, Muro I, Chen CH, Seol JH, Yoo SJ, Guo M, Park JM, Hay BA (2007) The Drosophila inhibitor of apoptosis (IAP) DIAP2 is dispensable for cell survival, required for the innate immune response to gram‐negative bacterial infection, and can be negatively regulated by the reaper/hid/grim family of IAP‐binding apoptosis inducers. J Biol Chem 282: 2056–2068 [DOI] [PubMed] [Google Scholar]
- Jiang H, Edgar BA (2011) Intestinal stem cells in the adult Drosophila midgut. Exp Cell Res 317: 2780–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Xu J, Yin MX, Lu Y, Hu L, Li P, Zhang P, Yuan Z, Ho MS, Ji H et al (2013) Brahma is essential for Drosophila intestinal stem cell proliferation and regulated by Hippo signaling. Elife 2: e00999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Patel PH, Kohlmaier A, Pavlovic B, Zhang C, Edgar BA (2017) Intestinal stem cell pool regulation in Drosophila . Stem Cell Reports 8: 1479–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuria S, Karpac J, Biteau B, Hwangbo D, Jasper H (2012) Notch‐mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLoS Genet 8: e1003045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil H, Bertrand MJ, Vandenabeele P, Widmann C (2014) Caspase‐3 and RasGAP: a stress‐sensing survival/demise switch. Trends Cell Biol 24: 83–89 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Du F, Chen SW, Nakasaki M, Rana I, Shih VFS, Hoffmann A, Jamora C (2015) Regulation and function of the Caspase‐1 in an inflammatory microenvironment. J Invest Dermatol 135: 2012–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Miguel‐Aliaga I (2013) The digestive tract of Drosophila melanogaster . Annu Rev Genet 47: 377–404 [DOI] [PubMed] [Google Scholar]
- Leulier F, Lhocine N, Lemaitre B, Meier P (2006) The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram‐negative bacterial infection. Mol Cell Biol 26: 7821–7831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jasper H (2016) Gastrointestinal stem cells in health and disease: from flies to humans. Dis Model Mech 9: 487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng FW, Biteau B (2015) A Sox transcription factor is a critical regulator of adult stem cell proliferation in the Drosophila intestine. Cell Rep 13: 906–914 [DOI] [PubMed] [Google Scholar]
- Meng FW, Rojas Villa SE, Biteau B (2020) Sox100B Regulates progenitor‐specific gene expression and cell differentiation in the adult Drosophila intestine. Stem Cell Reports 14: 226–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N (2006) Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439: 475–479 [DOI] [PubMed] [Google Scholar]
- Miguel‐Aliaga I, Jasper H, Lemaitre B (2018) Anatomy and physiology of the digestive tract of Drosophila melanogaster . Genetics 210: 357–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles WO, Dyson NJ, Walker JA (2011) Modeling tumor invasion and metastasis in Drosophila . Dis Model Mech 4: 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro I, Monser K, Clem RJ (2004) Mechanism of Dronc activation in Drosophila cells. J Cell Sci 117: 5035–5041 [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A (2006) The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439: 470–474 [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Petritsch C, Wen H, Jan L, Jan YN, Lu B (2011) Dronc caspase exerts a non‐apoptotic function to restrain phospho‐Numb‐induced ectopic neuroblast formation in Drosophila . Development 138: 2185–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish AB, Freel CD, Kornbluth S (2013) Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 5: a008672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff T, Antonello ZA, Ballesta‐Illan E, Mira L, Sala S, Navarro M, Martinez LM, Dominguez M (2019) Notch and EGFR regulate apoptosis in progenitor cells to ensure gut homeostasis in Drosophila . EMBO J 38: e101346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabinina O, Potter CJ (2016) The Q‐System: a versatile expression system for Drosophila . Methods Mol Biol 1478: 53–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Oliver H, Zou H, Chen P, Wang X, Abrams JM (1999) Dark is a Drosophila homologue of Apaf‐1/CED‐4 and functions in an evolutionarily conserved death pathway. Nat Cell Biol 1: 272–279 [DOI] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) Image J2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: 529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salle J, Gervais L, Boumard B, Stefanutti M, Siudeja K, Bardin AJ (2017) Intrinsic regulation of enteroendocrine fate by Numb. EMBO J 36: 1928–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al (2012) Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott S, Ambrosini A, Barbaste A, Benassayag C, Gracia M, Proag A, Rayer M, Monier B, Suzanne M (2017) A fluorescent toolkit for spatiotemporal tracking of apoptotic cells in living Drosophila tissues. Development 144: 3840–3846 [DOI] [PubMed] [Google Scholar]
- Shinoda N, Hanawa N, Chihara T, Koto A, Miura M (2019) Dronc‐independent basal executioner caspase activity sustains Drosophila imaginal tissue growth. Proc Natl Acad Sci USA 116: 20539–20544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silke J, Meier P (2013) Inhibitor of apoptosis (IAP) proteins‐modulators of cell death and inflammation. Cold Spring Harb Perspect Biol 5: a008730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula SM, Datta P, Kobayashi M, Wu JW, Fujioka M, Hegde R, Zhang Z, Mukattash R, Fernandes‐Alnemri T, Shi Y et al (2002) sickle, a novel Drosophila death gene in the reaper/hid/grim region, encodes an IAP‐inhibitory protein. Curr Biol 12: 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H (2008) Regulation of apoptosis in Drosophila . Cell Death Differ 15: 1132–1138 [DOI] [PubMed] [Google Scholar]
- Suzanne M, Steller H (2009) Letting go: modification of cell adhesion during apoptosis. J Biol 8: 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Zhao Y, Buchon N, Engstrom Y (2018) The POU/Oct transcription factor nubbin controls the balance of intestinal stem cell maintenance and differentiation by isoform‐specific regulation. Stem Cell Reports 10: 1565–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver BP, Weaver YM, Omi S, Yuan W, Ewbank JJ, Han M (2020) Non‐canonical caspase activity antagonizes p38 MAPK stress‐priming function to support development. Dev Cell 53: 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JP, Karres JS, Ogdahl JL, Zhou L, Schwartz LM, Nambu JR (2002) Drosophila sickle is a novel grim‐reaper cell death activator. Curr Biol 12: 131–135 [DOI] [PubMed] [Google Scholar]
- Xu D, Li Y, Arcaro M, Lackey M, Bergmann A (2005) The CARD‐carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila . Development 132: 2125–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Wang C, Shen X, Yu Y, Rui Y, Zhang D, Zhou Z (2013) Apoptotic block in colon cancer cells may be rectified by lentivirus mediated overexpression of caspase‐9. Acta Gastroenterol Belg 76: 372–380 [PubMed] [Google Scholar]
- Xu DC, Arthurton L, Baena‐Lopez LA (2018) Learning on the fly: the interplay between caspases and cancer. Biomed Res Int 2018: 5473180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Chauhan C, Hou SX (2010) Characterization of midgut stem cell‐ and enteroblast‐specific Gal4 lines in Drosophila . Genesis 48: 607–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Hou SX (2015) Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development 142: 644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Kondo S, Ha N, Boquete JP, Brunner M, Ueda R, Lemaitre B (2015) Accumulation of differentiating intestinal stem cell progenies drives tumorigenesis. Nat Commun 6: 10219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Boquete JP, Lemaitre B (2017) A genetic framework controlling the differentiation of intestinal stem cells during regeneration in Drosophila . PLoS Genet 13: e1006854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick RK, Ohlstein B, Klein OD (2019) Intestinal renewal across the animal kingdom: comparing stem cell activity in mouse and Drosophila . Am J Physiol Gastrointest Liver Physiol 316: G313–G322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
Data Availability Statement
All the raw images and data sets use for quantification purposes in the manuscript will be distributed upon reasonable request. The new fly strains generated for this manuscript will be deposited in the Bloomington Drosophila Stock Center (https://bdsc.indiana.edu/) and distributed upon request whilst the deposit is processed. This study includes no data deposited in external repositories.


