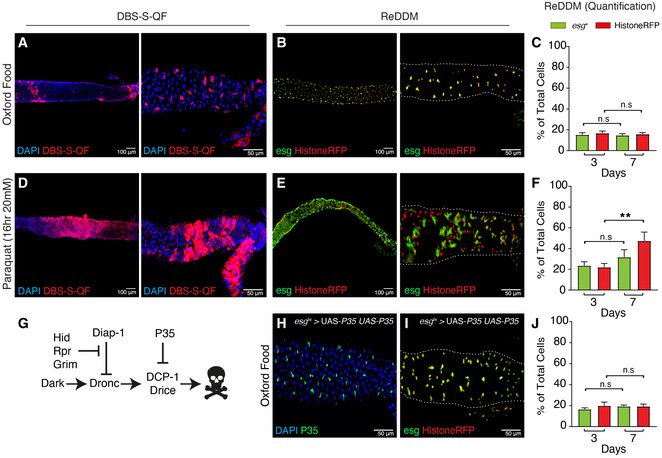
A representative example of a 7 days (7d) old adult female posterior midgut at low (A) and high magnification (B, Region 4‐Region 5) showing the initiator caspase reporter DBS‐S‐QF (Red, immunostaining anti‐HA). Flies were reared under experimental regime that protects the intestinal epithelial integrity (intestine reared in Oxford Medium and flies transferred every 2 days into vials with fresh food, see Fig
EV1) at 25°C. The image on the left was acquired at low magnification with the 10× objective, whilst the right panel was acquired using the 40×. The left and right images in all examples correspond to independent intestines of the same genotype and treatment. Genotype:
y1 w1118 UAS
‐mCD8::GFP.L QUAS
‐mtdTomato‐3xHA Act‐DBS‐S‐QF (BL83131).
A representative example of a 7‐d intestine reared at 29°C in experimental conditions that protect the epithelial integrity and labelled with ReDDM cell lineage tool. esg (green cytoplasmic signal) labels intestinal progenitor cells, whilst Histone‐RFP (red nuclear signal) acts as a semi‐permanent marker that allows the visualisation of differentiated cells. Note the extensive overlap between the two markers and the absence of differentiated cells only showing the Histone‐RFP labelling, as an indication of negligible epithelial turnover. The image on the left was acquired at low magnification with the 10× objective, whilst the right panel was acquired using the 40×. White dotted line outlines the gut using DAPI staining (not shown) as a reference. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP (Irene Miguel Aliaga)/Cyo; UAS‐Histone‐RFP (BL7019) TubG80ts (BL7019).
Quantification of intestinal cell subpopulations labelled with ReDDM system at high magnification (GFP and Histone‐RFP) and different time points post‐ReDDM activation (3 and 7 days) in experimental conditions that protect the epithelial integrity; note that none of the cell populations in the gut (GFP (n.s. no significant, P = 0.5267) or Histone‐RFP (n.s., P = 0.2752) significantly increase in number overtime (Quantifications were made using N ≥ 2 biological replicates, total number of guts analysed at 3 days n = 34 and at 7 days n = 45; Mann–Whitney, C). Error bars represent standard error of the mean. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP (Irene Miguel Aliaga)/ Cyo; UAS‐Histone‐RFP (BL7019) TubG80ts (BL7019).
A representative example of an adult female posterior midgut at low (A) and high magnification (B, Region 4‐Region 5) showing the initiator caspase reporter DBS‐S‐QF (Red, immunostaining anti‐HA) after 16 h of paraquat treatment in Oxford food at 25°C; note the expansion of the labelling with DBS‐S‐QF to large intestinal cells (ECs) (compare D with A). The image on the left was acquired at low magnification with the 10× objective, whilst the right panel was acquired using the 40×. White dotted line outlines the gut using DAPI staining as a reference. Genotype: y1 w1118 UAS‐mCD8::GFP.L QUAS‐mtdTomato‐3xHA Act‐DBS‐S‐QF (BL83131).
ReDDM lineage‐tracing in an adult intestine reared in Oxford Medium and paraquat (20 mM) during 16 h at 29°C; note the abundance of Histone‐RFP cells without GFP signal, as an indication of epithelial damage and subsequent differentiation of progenitor cells. The image on the left was acquired at low magnification with the 10× objective, whilst the right panel was acquired using the 40×. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP (Irene Miguel Aliaga)/Cyo; UAS‐Histone‐RFP (BL7019) TubG80ts (BL7019).
Quantification of ReDDM labelling at high magnification after paraquat treatment; note the statistically significant increase (**P = 0.0099) of Histone‐RFP expressing cells without GFP signal (Quantifications were made using N ≥ 2 biological replicates; Unpaired two‐tailed t‐test, 3d n = 9, 7d n = 7). Error bars represent standard error of the mean. Genotype: w1118; esg‐Gal4 UAS‐CD8‐GFP (Irene Miguel Aliaga)/Cyo; UAS‐Histone‐RFP (BL7019) TubG80ts (BL7019).
A simplified depiction of the apoptotic pathway in Drosophila. The pro‐apoptotic factors: Hid, Grim and Reaper, stimulate the formation of the apoptosome (Dark and Dronc), resulting in the activation of the effector caspases (Drice & DCP‐1) and cell death. Overexpression of the P35 baculoviral protein inhibits the activity of the effector caspases and cell death.
Representative example of an intestine 7d old expressing two copies of the effector caspase inhibitor P35 under the regulation of esg‐gal4 (green immunostaining with antibody against P35) at 29°C. Genotype: w
1118; esg‐Gal4 UAS‐CD8‐GFP/ UAS‐P35 (BL5072); UAS‐P35 (BL5073)/+.
ReDDM lineage‐tracing system in a Drosophila intestine expressing two copies of the effector caspase inhibitor P35 under the regulation of esg‐Gal4 and experimental conditions that protect the epithelial integrity at 29°C. Genotype: w
1118; esg‐Gal4 UAS‐CD8‐GFP/ UAS‐P35 (BL5072); UAS‐Histone‐RFP TubG80ts/ UAS‐P35 (BL5073).
ReDDM quantification corresponding to the intestines described in (I); no significant increase in either esg (n.s., P = 0.1352) or Histone‐RFP (n.s., P = 0.9801) cell number is observed (Quantifications were made using N ≥ 2 biological replicates; unpaired two‐tailed t‐test, 3d n = 12, 7d n = 11). Error bars represent standard error of the mean. DAPI (blue) labels the nuclei in panels A, D and H. Genotype: w
1118; esg‐Gal4 UAS‐CD8‐GFP/ UAS‐P35 (BL5072); UAS‐Histone‐RFP TubG80ts/ UAS‐P35 (BL5073).