Abstract
Background
Nationwide studies documenting temporal trends in permanent pacemaker implantation (PPMI) following transcatheter aortic valve replacement (TAVR) are limited.
Methods and Results
We selected patients who underwent TAVR between 2012 and 2017 in the National Readmission Database. The primary end point was the 6‐year trend in post‐TAVR PPMI at index hospitalization and at 30, 90, and 180 days after discharge. The secondary end point was the association between PPMI and in‐hospital mortality, stroke, cost, length of stay, and disposition. Among the 89 202 patients who underwent TAVR, 77 405 (86.8%) with no prior pacemaker or defibrillator were included. Patients who required PPMI had a higher prevalence of atrial fibrillation (43.6% versus 38.7%, P<0.001) and conduction abnormalities (28.4% versus 15.3%, P<0.001). The incidence of PPMI during index admission increased from 8.7% in 2012 to 13.2% in 2015, and then decreased to 9.6% in 2017. The incidence of inpatient PPMI within 30 days after discharge increased from 0.5% in 2012 to 1.25% in 2017 (P trend<0.001). Inpatient PPMI beyond 30 days remained rare (<0.5%) during the study period. After risk adjustment, PPMI was not associated with in‐hospital mortality or stroke but was associated with increased nonhome discharge, longer hospitalization, and higher cost. The incremental expenditure associated with post‐TAVR PPMI during index admission increased from $9.6 million to $72.2 million between 2012 and 2017.
Conclusions
After an upward trend, rates of PPMI after TAVR in the United States stabilized at ~10% in 2016 to 2017, but there was a notable increase in PPMI within 30 days after the index admission. PPMI was not associated with increased in‐hospital morbidity or mortality but led to longer hospitalization, higher cost, and more nonhome discharges.
Keywords: aortic stenosis, cardiac resynchronization therapy, heart block, permanent pacemaker implantation, transcatheter aortic valve replacement
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Electrophysiology
Abbreviations
- CRT
cardiac resynchronization therapy
- LOS
length of stay
- NRD
National Readmission Database
- PPMI
permanent pacemaker implantation
- TAVR
transcatheter aortic valve replacement
- THV
transcatheter heart valve
Clinical Perspective
What Is New?
About 1 in 10 patients undergoing transcatheter aortic valve replacement in the United States receive a permanent pacemaker before discharge and that number has not decreased over time.
There is a temporal increase in the incidence of permanent pacemaker implantation after discharge among patients undergoing transcatheter aortic valve replacement.
What Are the Clinical Implications?
The rising incidence of postdischarge conduction abnormalities requiring pacemaker after transcatheter aortic valve replacement suggests the need for careful monitoring, especially with the increasing practice of early postprocedural dismissal.
More research is needed to understand the long‐term clinical and economic impact of permanent pacemaker after transcatheter aortic valve replacement in light of the expanding indications for this therapy.
Conduction disturbances requiring permanent pacemaker implantation (PPMI) are known complications of aortic valve interventions. 1 , 2 , 3 With surgical aortic valve replacement, the incidence of postoperative PPMI in the United States has been stable around 5% to 6% in the past 2 decades. 1 , 2 , 4 In contrast, the incidence of PPMI after transcatheter aortic valve replacement (TAVR) has been both variable and dynamic over time. In the earliest randomized trial of TAVR, PPMI was only required postoperatively in 3.4% of patients. 5 Subsequent trials have shown higher rates of PPMI after TAVR, especially with self‐expanding valves. 6 A survey of the early commercial TAVR experience in the United States between 2011 and 2014 documented a 30‐day incidence of PPMI following TAVR of 6.7%. 7 However, TAVR practice in the United States has evolved markedly since 2014 with the emergence of second‐ and third‐generation transcatheter heart valves (THV), and the recognition of anatomical and procedural factors that increase the risk of PPMI. 2 , 8 , 9 , 10 , 11 Although there is a plethora of literature on post‐TAVR PPMI, nationwide data on the temporal incidence and outcomes of PPMI after TAVR are limited. 12 To address this knowledge gap, we used a national representative database to assess the temporal change in the incidence, timing, and outcomes of PPMI following TAVR between 2012 and 2017.
Methods
Data obtained from the National Readmission Database (NRD) could not be shared directly by the authors, but requests to access the NRD data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Healthcare Cost and Utilization Project (HCUP; https://www.hcup-us.ahrq.gov/team/NationwideDUA.jsp).
Study Data
The NRD was used to derive patient‐relevant information. The NRD is a publicly available, all‐payer data set of inpatient stays in hospitals from 27 geographically dispersed states. These hospitalizations account for 60% of all hospitalizations in the United States annually. The NRD also contains verified patient linkage numbers that can be used to track readmissions across hospitals for individual patients within the same calendar year. The institutional review board exempted the study because it utilizes public deidentified data. NRD is a convenience sample that is drawn from HCUP State Inpatient Databases and is poststratified to reflect the target universe of inpatient discharges treated at community hospitals in the United States that are not rehabilitation or long‐term acute care facilities. The NRD database includes variables for sampling weights (DISCWT), hospital clusters (HOSP_NRD), and stratification (NRD_STRATUM).
Study Population
Adult patients (aged >18 years) who underwent TAVR between January 1, 2012, and December 31, 2017, were identified in the NRD using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9-CM) and tenth revision (ICD‐10-CM) codes (Table S1). To calculate the rate of new PPMI after TAVR during the index admission, patients with prior permanent pacemaker or internal cardioverter‐defibrillator were excluded (Figure 1). In addition, to calculate the rate of PPMI after discharge, the following subgroups of patients were excluded: TAVRs performed in December were excluded from calculating 30‐day postdischarge rate of PPMI; TAVRs performed in October through December were excluded from calculating 90‐day postdischarge rate of PPMI; TAVRs performed in July through December were excluded from calculating 180‐day postdischarge rate of PPMI. This approach was necessary because the NRD does not track patients across consecutive years.
Figure 1. Study flow chart.
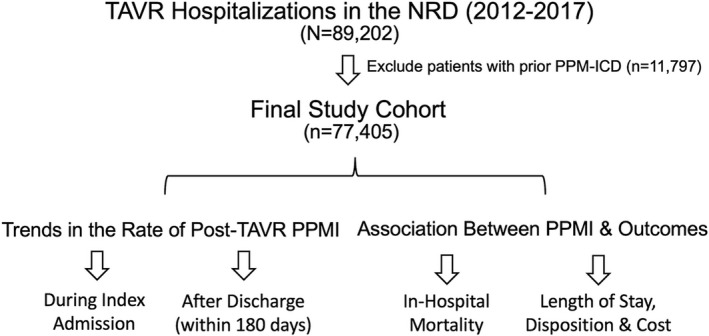
ICD indicates internal cardioverter‐defibrillator; NRD, National Readmission Database; PPM, permanent pacemaker; PPMI, permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Study End Point
The primary end point of this study was the temporal trend in the incidence of post‐TAVR PPMI during the index hospitalization and at 30, 90, and 180 days beyond discharge. The secondary end points were the temporal trends in the association between PPMI and in‐hospital mortality, stroke, cost, length of stay (LOS), and discharge disposition. This association was assessed across 3 equally divided time periods (2012–2013, 2014–2015, 2016–2017). These periods roughly corresponded with the timeline of various generations of THVs introduced into the commercial market in the United States. We also described the baseline characteristics of patients who required PPMI after TAVR versus those who did not, trends of cardiac resynchronization therapy (CRT) utilization, and trends of annual expenditure related to post‐TAVR PPMI in the United States.
Statistical Analysis
Descriptive statistics were presented as frequencies with percentages for categorical variables. Means, standard deviations, medians, and interquartile ranges were reported for continuous variables. Patient demographics, comorbidities, hospital characteristics, and in‐hospital outcomes were compared between patients who needed PPMI after TAVR and those who did not, using the Pearson χ2 test for categorical variables and independent‐samples t test for continuous variables. We used the Cochrane‐Armitage test for trend analyses of categorical variables (eg, incidence of post‐TAVR PPMI over years) and as a sensitivity analysis for trends, and a logistic regression model was used as well; PPMI status was the outcome, and the calendar year was included in the model as a continuous variable. To assess the association between PPMI and in‐hospital outcomes, we excluded patients who died within 24 hours of the TAVR procedure and those in whom the procedure was converted to open surgery. Excluding deaths that happened during the TAVR procedure or shortly after was necessary to avoid selection bias because the majority of the patients who died did not get a chance to receive a permanent pacemaker. To account for the differences in baseline characteristics between patients who received a PPMI and those who did not, risk adjustment for clinical risk factors and hospital characteristics was performed using mixed‐effects logistic regression survey models. The Stata survey command was used in these models. This command considered the NRD sampling design (strata, hospital clusters, and poststratification weights). Year was added to NRD strata and clusters. Dependent (outcome) variables included death, ischemic stroke, nonhome discharge, LOS, and costs. Mixed‐effects models were used because of the clustering of the observations within hospitals. Logistic regression was used for binary outcomes (death, ischemic stroke, and nonhome discharge), and linear regression was used for continuous outcomes (LOS and costs). The independent variable of interest was the PPMI status. The models were adjusted for the following confounders, which are listed in Table S2: age, sex, diabetes mellitus, hypertension, peripheral vascular disease, carotid artery disease, chronic pulmonary disease, home oxygen use, prior sternotomy, anemia, prior history of stroke, atrial fibrillation, chronic kidney disease, smoking, dementia, Parkinson disease, calorie protein malnutrition, malignancy, obesity, liver cirrhosis, coronary artery disease, teaching status of the hospital, Medicare or Medicaid insurance, hospital bed size, household income quartile, elective admission, and TAVR access. NRD discharge weights were used to calculate national estimates. Covariates included in the risk adjustment models are listed in Table S2. Unadjusted and adjusted P values are reported. Hospital charges were obtained from the hospital accounting reports collected by the Centers for Medicare and Medicaid Services. The HCUP Cost‐to‐Charge ratio file was then used to calculate costs by multiplying the charges by the cost‐to‐charge ratio. Cost data were additionally adjusted for inflation using the Consumer Price Index (www.bls.gov); costs are reported in 2017 US dollars. Costs and LOS have a skewed distribution, so median and interquartile range are reported. LOS and total costs were transformed to the normal log scale and included in the multivariate linear model. However, given the large sample size, parametric tests (mean and linear regression) were also used as a sensitivity analysis based on the suggestion of Thompson and Barber 13 that these tests are also robust. A multivariable mixed‐effects linear regression model was used to estimate the adjusted coefficient of cost, which can be interpreted as the adjusted difference in the average cost between both groups (OR indicates odds ratio): Percentagecase = (OR × Percentagecontrol)/(1 + OR × Percentagecontrol − Percentagecontrol). Adjusted percentages were also calculated using the rate ratios obtained from a multivariable Poisson regression with robust error variance. 14 , 15 , 16 , 17 To evaluate the associations of PPMI–LOS and PPMI–cost across the 3 periods, we reran 4 models and included the interaction term between period and PPMI as an independent variable in each of them: (1) linear regression of LOS over PPMI status, period, and the interaction term between both; (2) linear regression of LOS over PPMI status, period, the interaction term, and all other confounders that are listed in Table S2; (3) linear regression of costs over PPMI status, period, and the interaction term between both; and (4) linear regression of the costs over PPMI status, period, the interaction term, and all other confounders that are listed in Table S2.
To calculate the total expenditure associated with PPMI during hospital admissions, we used the national weighted estimates of TAVR procedures and multiplied them by the mean difference in the cost of hospitalization between the PPMI and no PPMI groups per year. Some data were missing, particularly for hospital size. The remaining variables were largely complete with <5% missing. Because hospital size was not the focus of this study, it was not imputed using complex statistical methods. A type I error of <0.05 was considered statistically significant. All analyses were performed using SPSS v24 (IBM Corp), Microsoft Excel (2010), and STATA v15.1 (StataCorp).
Results
A total of 89 202 unique hospitalizations for TAVR were identified in the NRD between January 1, 2012, and December 31, 2017. After excluding 11 797 patients with prior pacemaker or internal cardioverter‐defibrillator, 77 405 were included in the assessment of the primary end point (Figure 1). Compared with patients who did not receive a PPMI, those who required post‐TAVR PPMI were older (81.7±7.4 versus 80.1±8.6, P<0.001), were more likely to be male (54.8% versus 51.9%, P<0.001), and had higher prevalence of atrial fibrillation (43.6% versus 38.7%, P<0.001) and baseline conduction abnormalities (18.6% versus 7.6%, P<0.001). They also had higher rates of hypertension, diabetes mellitus, anemia, and chronic kidney disease (Table 1). The temporal change in baseline characteristics in both groups is shown in Table S3.
Table 1.
Baseline Characteristics of Patients With and Without Post‐TAVR PPMI
| Patient Characteristic | TAVR Without PPMI (n=69 527) | TAVR With PPMI (n=7878) | P Value |
|---|---|---|---|
| Age, y, mean±SD | 80.1±8.6 | 81.7±7.4 | <0.001 |
| Male sex | 51.9 | 54.8 | <0.001 |
| Diabetes mellitus | 37.7 | 39.9 | <0.001 |
| Hypertension | 62.1 | 64.9 | <0.001 |
| Peripheral vascular disease | 23.1 | 22.5 | 0.20 |
| Carotid artery disease | 2.1 | 1.7 | 0.06 |
| Chronic pulmonary disease | 27.0 | 26.8 | 0.65 |
| Home oxygen | 5.6 | 5.4 | 0.57 |
| Prior sternotomy | 19.9 | 19.5 | 0.43 |
| Anemia | 24.2 | 26.3 | <0.001 |
| Prior history of stroke | 9.9 | 9.1 | 0.02 |
| Atrial fibrillation | 38.7 | 43.6 | <0.001 |
| Conduction disorders | 18.6 | 57.6 | <0.001 |
| Chronic kidney disease | 34.5 | 38.6 | <0.001 |
| Dialysis dependence | 2.5 | 3.2 | <0.001 |
| Smoking | 11.7 | 12.0 | 0.54 |
| Dementia | 4.8 | 6.1 | <0.001 |
| Parkinson disease | 1.3 | 1.2 | 0.40 |
| Calorie protein malnutrition | 2.2 | 2.4 | 0.37 |
| Malignancy | 5.7 | 5.8 | 0.82 |
| Obesity | 17.2 | 17.8 | 0.20 |
| Liver cirrhosis | 0.5 | 0.4 | 0.01 |
| Coronary artery disease | 67.5 | 68.6 | 0.04 |
| Teaching hospital | 54.0 | 60.1 | <0.001 |
| Medicare/Medicaid insurance | 91.7 | 93.1 | <0.001 |
| Large hospital bed size | 78.1 | 80.5 | 0.001 |
| Lowest quartile household income | 19.7 | 16.8 | <0.001 |
| Elective admission | 78.6 | 75.6 | <0.001 |
| Transfemoral TAVR access | 94.3 | 95.6 | <0.001 |
Data are percentages except as noted. PPMI indicates permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Incidence of Post‐TAVR PPMI
The incidence of post‐TAVR PPMI during the index admission increased from 8.7% in 2012 to a peak of 13.2% in 2015 before decreasing to 9.6% in 2017 (P trend<0.001, logistic regression model P=0.045) (Figure 2). A minority of patients undergoing PPMI received CRT, and this did not change significantly over time (6.0% in 2012 to 6.4% in 2017, P trend=0.66) (Figure 3). Notably, the incidence of inpatient post‐TAVR PPMI within 30 days of hospital discharge increased significantly from 0.5% in 2012 to 1.25% in 2017 (P trend<0.001) (Figure 4). This finding correlated with a temporal increase in the proportion of 30‐day readmissions for conduction disturbances (among all 30‐day readmissions post‐TAVR) from 4.1% in 2012 to 12.0% in 2017 (Figure 5). PPMI beyond 30 days was uncommon overall (0.29% between 31 and 90 days, and 0.31% between 91 and 180 days), with no statistically significant temporal change (P trend=0.07 and P trend=0.89, respectively) (Figure 2).
Figure 2. Temporal trend in the incidence of post‐TAVR PPMI during the index admission between 2012 and 2017.
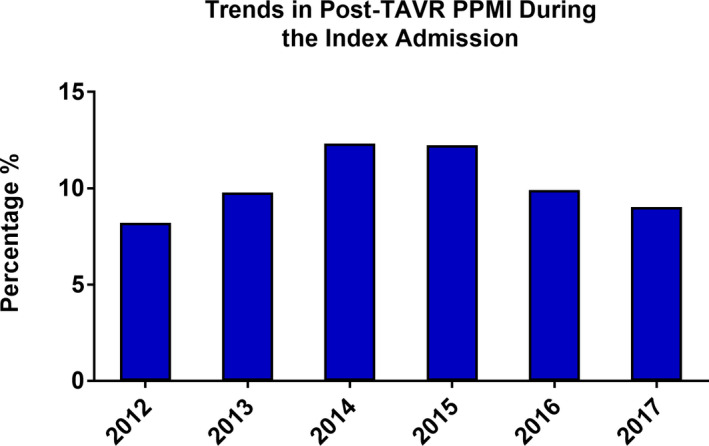
PPMI indicates permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Figure 3. Utilization of CRT among patients undergoing post‐TAVR PPMI in the United States between 2012 and 2017.
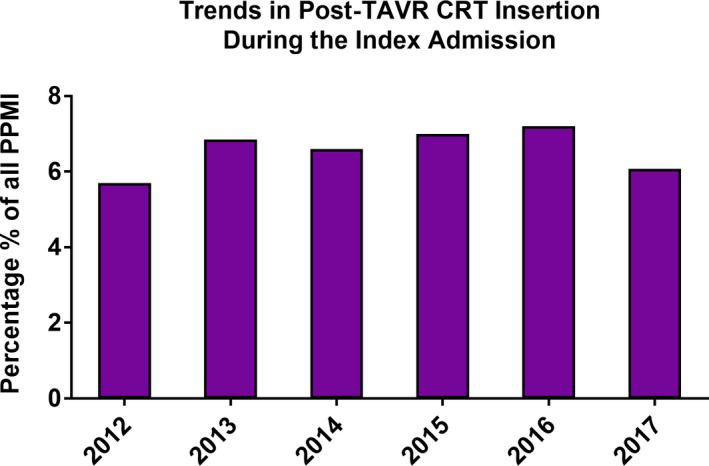
CRT indicates cardiac resynchronization therapy; PPMI, permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Figure 4. Temporal trend in the incidence of inpatient post‐TAVR PPMI after discharge between 2012 and 2017.
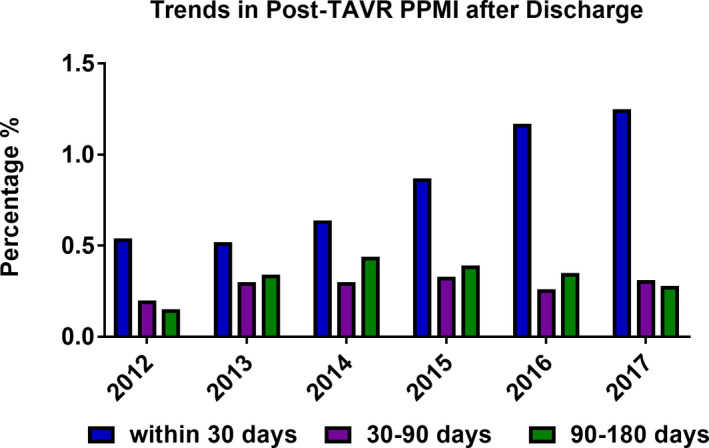
PPMI indicates permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Figure 5. Proportion of 30‐day readmissions for PPMI among all 30‐day readmissions after TAVR.
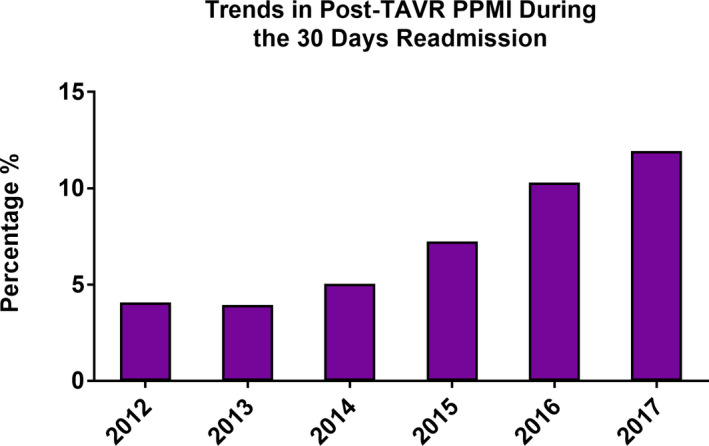
PPMI indicates permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Association Between PPMI During the Index Admission and Outcomes
Patients who underwent PPMI had lower unadjusted in‐hospital mortality compared with those who did not (1.8% versus 2.6%, P<0.001). However, they had higher unadjusted incidence of acute kidney injury, new dialysis, blood transfusion, tamponade, and mechanical ventilation (Table 2). PPMI was also associated with higher cost, longer hospitalizations, and higher rates of nonhome discharge (Table 2), and these associations were consistent over time (Table S4). Differences in baseline conduction disorder between patients with and without post‐TAVR PPMI are listed in Table S5. After adjustment for risk factors and hospital characteristics, and exclusion of patients who had emergent surgery or who died within 24 hours of the procedure, PPMI was not associated with higher in‐hospital mortality; the OR ranged between 0.61 (95% CI, 0.39–0.97) in 2016 to 2017 and 0.88 (95% CI, 0.67–1.16) in 2014–2015. Moreover, there was no association between PPMI and the incidence of acute ischemic stroke (Table 3). Nevertheless, PPMI was associated with significantly higher odds of nonhome discharges. The adjusted OR for the association between PPMI and nonhome discharge increased from 1.48 (95% CI, 1.16–1.89) in 2012 through 2013 to 1.83 (95% CI, 1.62–2.07) in 2016 through 2017. In addition, the associations between PPMI and LOS and cost remained consistent over time (Table 4). The total estimated incremental expenditure associated with post‐TAVR PPMI increased from $9.6 million in 2012 to $72.2 million in 2017 (P trend<0.001) (Figure 6). The P values for the interaction terms were nonsignificant in all models. These findings suggest that the PPMI–LOS and PPMI–cost associations were consistent across periods.
Table 2.
Outcomes of Patients With and Without Post‐TAVR PPMI
| In‐Hospital Outcome | TAVR Without PPMI (n=69 527) | TAVR With PPMI (n=7878) | P Value |
|---|---|---|---|
| Death | 2.6 | 1.8 | <0.001 |
| Acute ischemia stroke | 1.2 | 1.2 | 0.55 |
| Acute kidney injury | 13.1 | 19.0 | <0.001 |
| AKI requiring dialysis | 1.0 | 1.2 | 0.048 |
| Blood transfusion | 12.0 | 15.1 | <0.001 |
| Vascular complication | 3.7 | 3.8 | 0.45 |
| Tamponade | 0.9 | 1.6 | <0.001 |
| Gastrointestinal bleed | 1.3 | 1.4 | 0.41 |
| Mechanical ventilation | 3.0 | 4.0 | <0.001 |
| Length of stay, median (IQR) | 3 (2–7) | 6 (4–9) | <0.001 |
| Hospital cost, USD, median (IQR) | 47 661 (37 304–62 244) | 63 471 (50 925–80 709) | <0.001 |
| Length of stay >5 d | 31.9 | 51.8 | <0.001 |
| Discharged to a facility | 16.6 | 27.3 | <0.001 |
Data are percentages except as noted. AKI indicates acute kidney injury; IQR, interquartile range; PPMI, permanent pacemaker implantation; TAVR, transcatheter aortic valve replacement; USD, US dollars.
Table 3.
Association Between Post‐TAVR PPMI and In Hospital Outcomes
| Outcome | 2012–2013 | 2014–2015 | 2016–2017 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Death | ||||||
| Unadjusted | 0.82 (0.55–1.24) | 0.35 | 0.87 (0.67–1.14) | 0.31 | 0.74 (0.54–1.02) | 0.062 |
| Adjusted | 0.79 (0.46–1.37) | 0.41 | 0.88 (0.67–1.16) | 0.36 | 0.61 (0.39–0.97) | 0.036 |
| Ischemic stroke | ||||||
| Unadjusted | 0.74 (0.41–1.3) | 0.32 | 0.81 (0.6–1.09) | 0.16 | 1.1 (0.78–1.6) | 0.53 |
| Adjusted | 0.76 (0.37–1.58) | 0.46 | 0.82 (0.61–1.11) | 0.21 | 1.39 (0.88–2.22) | 0.16 |
| Nonhome discharge | ||||||
| Unadjusted | 1.56 (1.29–1.88) | <0.001 | 1.55 (1.39–1.73) | <0.001 | 1.98 (1.83–2.14) | <0.001 |
| Adjusted | 1.48 (1.16–1.89) | 0.001 | 1.51 (1.35–1.69) | <0.001 | 1.86 (1.64–2.09) | <0.001 |
OR indicates odds ratio; PPMI, permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Table 4.
Association Between Post‐TAVR PPMI and Cost and Length of Stay
| Outcome | 2012–2013 | 2014–2015 | 2016–2017 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPMI | No PPMI | P Value | Adjusted Coefficient | P Value | PPMI | No PPMI | P value | Adjusted Coefficient | P Value | PPMI | No PPMI | P Value | Adjusted Coefficient | P Value | |
| LOS,* median (IQR) | 8 (6, 14) | 6 (4, 11) | <0.001 | NA | NA | 6 (4, 10) | 4 (3, 8) | <0.001 | NA | NA | 5 (3, 8) | 3 (2, 5) | <0.001 | NA | NA |
| LOS, mean (95% CI) | 11.6 (10.8–12.5) | 9.4 (9–9.9) | <0.001 | 1.9 (1.2–2.5) | <0.001 | 9.1 (8.5–9.7) | 7.3 (6.9–7.6) | <0.001 | 1.7 (1.3–2.1) | <0.001 | 7 (6.6–7.4) | 4.8 (4.7–5) | <0.001 | 1.8 (1.3–2.2) | <0.001 |
| LOS (normal log scale) | 2.08 (1.79–2.64) | 1.79 (1.38–2.39) | <0.001 | 0.26 (0.21–0.3) | <0.001 | 1.79 (1.39–2.3) | 1.39 (1.1–2.08) | <0.001 | 0.28 (0.25–0.32) | <0.001 | 1.61 (1.1–2.08) | 1.1 (0.69–1.61) | <0.001 | 0.4 (0.35–0.45) | <0.001 |
| Cost,** median (IQR) | 67 744 (54 252, 88 916) | 52 117 (40 445, 69 401) | <0.001 | NA | NA | 64 445 (51 458, 82 614) | 49 864 (39 580, 65 625) | <0.001 | NA | NA | 62 169 (50 367, 78 344) | 45 912 (36 120, 59 372) | <0.001 | NA | NA |
| Cost, mean (95% CI) | 74 399 (70 570–78 228) | 58 905 (57 102–60 707) | <0.001 | 13 094 (9548–16 639) | <0.001 | 68 460 (65 687–71 233) | 56 171 (54 410–57 933) | <0.001 | 12 396 (10 613–14 178) | <0.001 | 67 068 (64 782–69 354) | 50 600 (49 140–52 059) | <0.001 | 15 368 (13 428–17 308) | <0.001 |
| Cost (normal log scale) | 11.19 (10.96–11.45) | 10.92 (10.67–11.2) | <0.001 | 0.23 (0.18–0.28) | <0.001 | 11.11 (10.88–11.36) | 10.85 (10.62–11.13) | <0.001 | 0.22 (0.2–0.25) | <0.001 | 11.08 (10.87–11.31) | 10.78 (10.54–11.04) | <0.001 | 0.27 (0.25–0.3) | <0.001 |
Clinical covariates used for adjustment are listed in Table S2. In addition, cost data were adjusted for inflation. IQR indicates interquartile range; LOS, length of stay; NA, nonapplicable; PPMI, permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Days.
US dollars.
Figure 6. Annual expenditure associated with PPMI during the index admission after TAVR.
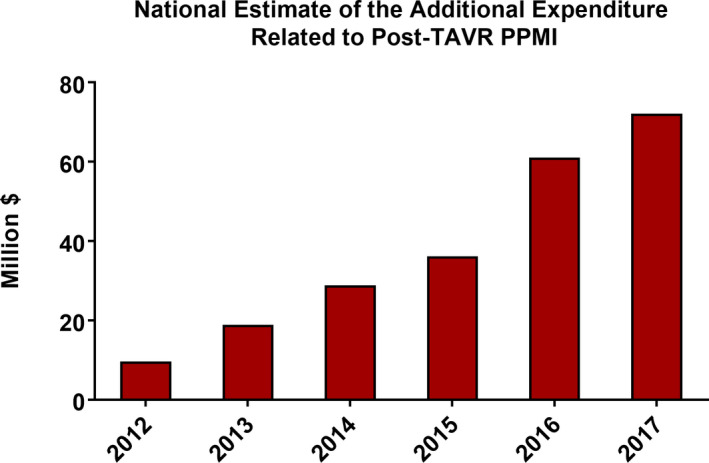
PPMI indicates permanent pacemaker implantation; and TAVR, transcatheter aortic valve replacement.
Discussion
The current investigation has 2 main findings. First, the incidence of post‐TAVR PPMI during the index admission increased between 2012 and 2015 but decreased to a stable rate of ≈10% afterward. In contrast, the need for PPMI within 30 days after discharge, although it remained uncommon, increased by >2‐fold between 2012 and 2017. Second, after risk adjustment, the need for PPMI after TAVR did not increase in‐hospital morbidity or mortality. However, it did substantially increase the LOS, cost, and the need for an intermediate care facility. The total national expenditure on post‐TAVR PPMI increased >7‐fold between 2012 and 2017.
The demonstrable benefit of TAVR across all patient risk categories expanded its role to the majority of patients with aortic stenosis. 2 , 6 However, concerns remain about unresolved issues with TAVR such as the attendant risk of needing PPMI. 18 Consequently, a growing number of publications concerning post‐TAVR PPMI are surfacing in the literature. 19 Although such studies have established the incidence, predictors, and outcomes of post‐TAVR PPMI, they included heterogenous or selected cohorts of patients and often reached contradictory conclusions. 20 , 21 , 22 , 23 , 24 Nationwide surveys of post‐TAVR PPMI remain scarce. The largest nationwide assessment of post‐TAVR PPMI, from the Transcatheter Valve Therapeutics (TVT) registry, provided key insights into the issue of post‐TAVR PPMI in the United States. 7 However, this study was limited to 9785 patients who received TAVR before September 2014, which does not represent contemporary practice. In addition, to our knowledge, no prior studies have assessed the timing of PPMI or the resource utilization and cost associated with it. Therefore, our study sought to reduce the knowledge gap on this increasingly important issue.
We found a temporal increase in the rate of post‐PPMI after TAVR between 2012 and 2015 followed by a decline to a stable rate of ≈10% in 2016 to 2017. This initial observed increase was likely related to the introduction of the CoreValve (Medtronic) and Sapien‐XT (Edwards Lifesciences) devices into the US market in January 2014 and June 2014, respectively. Both valves were associated with higher PPMI rates compared with Sapien, which was the only THV available in the United States before 2014. 7 , 25 Although speculative, the later decrease may have been related to the stabilization of the US market with a larger share of balloon versus self‐expandable THVs and the adoption of procedural strategies that have been shown to reduce the risk of PPMI (eg, higher THV implantation). 9 , 26 The rates of PPMI after TAVR may have surged again in 2018 to 2019, given the dynamic change in the types and market share of THVs and the variable risk of PPMI associated with those valves. 27 , 28 , 29 , 30 For example, the approval of the Lotus THV (Boston Scientific) in April 2019 might affect the national post‐TAVR PPMI, given that Lotus was associated with a 2‐year PPMI rate of 41.7% in the pivotal trial (Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus™ Valve System ‐ Randomized Clinical Evaluation). 31 These findings emphasize the need for continuous assessment of PPMI after TAVR, especially with the rising number of younger and low‐risk patients receiving TAVR.
The timing of PPMI after TAVR has been an area of interest and ongoing debate, but little relevant data exist. The median time to PPMI after TAVR is ≈3 days in most studies. 22 , 32 In the TVT registry, >95% of PPMIs were performed within 14 days after TAVR, but the percentage of PPMIs during subsequent hospitalizations was not reported. 7 The distinction between PPMI during the index admission versus after discharge is critical in light of the growing literature on delayed post‐TAVR heart block that is detected after discharge. 22 , 33 , 34 Our study showed a significant temporal increase in the incidence of PPMI within 30 days after discharge (0.5% in 2012 to 1.25% in 2017). Although small overall, this increase represented a tripling of the percentage of 30‐day readmissions for PPMI (out of all 30‐day readmissions) from 4% to 12%. Whether this is related to the shorter LOS in contemporary TAVR practice (ie, missed opportunity to detect late conduction disturbances in the hospital) or represents a device‐specific effect, with some THVs tending to cause late conduction abnormalities, remains to be studied. 32 Nonetheless, these observations should raise awareness of delayed heart block after TAVR and prompt further investigations of its mechanisms and mitigation strategies such as remote monitoring with implantable or wearable devices.
The association between PPMI after TAVR and clinical outcomes has been studied previously. 7 , 21 , 35 , 36 , 37 , 38 , 39 Our population‐based analysis confirms the findings of those prior smaller studies that showed no association between post‐TAVR PPMI and short‐term mortality or stroke. This observation was consistent across the study period. Unfortunately, Our study is unable to assess long‐term impact of PPMI after TAVR because of the lack of long‐term follow‐up in the NRD. Nonetheless, the associations between PPMI and long‐term mortality and heart failure hospitalizations after TAVR are well established. 7 , 36 , 37 , 38 , 39 A potentially relevant observation that may be pertinent to long‐term outcomes in our study is the utilization of CRT. Cardiac resynchronization has been suggested as a potential strategy to reduce the negative long‐term impact of PPMI among patients as PPMI dependability has increased. 21 , 40 We found that a very small percentage (≈6%) of patients undergoing PPMI following TAVR receive CRT, and this percentage was consistent over time. Whether this is due to the patient’s specific characteristics, the lack of data supporting the role of CRT among patients who receive TAVR, or the potential incremental financial loss with CRT, given that PPMI after TAVR are not separately reimbursable, remains unknown. With the continuous expansion of TAVR to lower risk and younger patients, the role of various pacing modalities (single‐chamber, wireless, and CRT pacemakers) needs to be further investigated.
The impact of PPMI on resource utilization and cost after TAVR is increasingly relevant in light of anticipated Medicare payment cuts and the growing number of TAVR procedures at the site level and nationally. 41 However, few data exist regarding the economic impact of post‐TAVR PPMI. Our study documented a substantial impact of post‐TAVR PPMI on LOS, cost, and discharge disposition, and this impact was consistent over time. Specifically, post‐TAVR PPMI was associated with a 48% to 86% increase in nonhome discharge, ≈2‐day increase in LOS, and a $12 000 to $15 000 incremental cost after adjustments for clinical covariates, hospital characteristics, and inflation. This cost increase translated into a cost of ≈$72 million in 2017 for index TAVR hospitalizations alone. The true overall cost associated with PPMI is likely higher because these cost figures do not include PPMIs at subsequent visits, the cost related to the increase in intermediate care facility utilization, and the increased number of heart failure hospitalizations that has been shown for patients with post‐TAVR PPMI in prior studies. 7 , 37 , 38 , 39 These data highlight the need for long‐term cost analyses, especially with the increasing number of TAVR procedures and the availability of various THVs with varied associated PPMI risks. 42
Limitations
First, the NRD is a claim‐based database. Data provided by the NRD are collected mainly for billing purposes and thus are prone to the pitfalls of under‐ or overcoding. However, coding of procedures, vital status, and major complications is essential for adequate reimbursement by hospitals and are less prone to major errors. Second, the NRD does not track patients across different years. Consequently, we are unable to identify patients who underwent subsequent PPMI during prolonged follow‐up. However, our findings suggest that the vast majority of PPMIs after TAVR occur either during the index admission or within 30 days after discharge; therefore, this limitation is unlikely to have a large impact on our results. Third, the NRD includes only inpatient visits, and PPMI that occurs during encounters that are coded as observational and/or outpatient are not captured. Fourth, the NRD does not contain essential granular data related to the electrophysiologic findings or the indication for the PPMI. As a result, description of post‐TAVR conduction abnormalities that might have led to the PPMI (eg, high degree of atrioventricular blockage, bifascicular or trifascicular block) could not be performed. In addition, some selection bias may exist because the threshold to proceed with PPMI may vary among different operators. However, this limitation also applies to all registry‐based studies addressing post‐TAVR PPMI. Fifth, data on valve type are not available in the NRD; therefore, we could not study the association between self‐ or balloon‐expandable valves and PPMI. Nonetheless, this association is well established in the literature. 43 Because our study did not aim to specifically assess predictors of PPMI after TAVR, we believe that this limitation has little impact on the overall findings. Further studies assessing the temporal impact of THV selection on PPMI incidence and outcomes are needed to address this issue.
Conclusions
The incidence of PPMI following TAVR in the United States increased significantly between 2012 and 2015 but stabilized at ≈10% afterward. However, a small but significant temporal increase in PPMI beyond the index TAVR admission was observed. PPMI was not associated with increased risk‐adjusted morbidity or mortality but led to longer hospitalizations, higher cost, and more nonhome discharges.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S5
(J Am Heart Assoc. 2020;9:e016685 DOI: 10.1161/JAHA.120.016685.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016685
For Sources of Funding and Disclosures, see page 9.
References
- 1. Moskowitz G, Hong KN, Giustino G, Gillinov AM, Ailawadi G, DeRose JJ Jr, Iribarne A, Moskowitz AJ, Gelijns AC, Egorova NN. Incidence and risk factors for permanent pacemaker implantation following mitral or aortic valve surgery. J Am Coll Cardiol. 2019;2607–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alkhouli M, Alqahtani F, Ziada KM, Aljohani S, Holmes DR, Verghese M. Contemporary trends in the management of aortic stenosis in the USA. Eur Heart J. 2020;921–928. [DOI] [PubMed] [Google Scholar]
- 3. Alkhouli M, Zack CJ, Sarraf M, Bashir R, Nishimura RA, Eleid MF, Nkomo VT, Sandhu GS, Gulati R, Greason KL, et al. Morbidity and mortality associated with balloon aortic valvuloplasty. A national perspective. Circ Cardiovasc Interv. 2017;e004481 DOI: 10.1161/CIRCINTERVENTIONS.116.004481. [DOI] [PubMed] [Google Scholar]
- 4. Chaker Z, Badhwar V, Alqahtani F, Aljohani S, Zack CJ, Holmes DR,Rihal CS, Alkhouli M. Sex differences in the utilization and outcomes of surgical aortic valve replacement for severe aortic stenosis. J Am Heart Assoc. 2017;e006370 DOI: 10.1161/JAHA.117.006370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;1597–1607. [DOI] [PubMed] [Google Scholar]
- 6. Khan SU, Riaz H, Khan MU, Zarak MS, Khan MZ, Khan MS, Sattur S, Desai MY, Kaluski E, Alkhouli M. Meta‐analysis of temporal and surgical risk dependent associations with outcomes after transcatheter versus surgical aortic valve implantation. Am J Cardiol. 2019;1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli S, Elgin E, Donato A. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2016;2189–2199. [DOI] [PubMed] [Google Scholar]
- 8. Kiani S, Kamioka N, Black GB, Lu MLR, Lisko JC, Rao B, Mengistu A, Gleason PT, Stewart JP, Caughron H, et al. Development of a risk score to predict new pacemaker implantation after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;2133–2142. [DOI] [PubMed] [Google Scholar]
- 9. Jilaihawi H, Zhao Z, Du R, Staniloae C, Saric M, Neuburger PJ, Querijero M, Vainrib A, Hisamoto K, Ibrahim H, et al. Minimizing permanent pacemaker following repositionable self‐expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;1796–1807. [DOI] [PubMed] [Google Scholar]
- 10. Ream K, Sandhu A, Valle J, Weber R, Kaizer A, Wiktor DM, Borne RT, Tumolo AZ, Kunkel M, Zipse MM, et al. Ambulatory rhythm monitoring to detect late high‐grade atrioventricular block following transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;2538–2547. [DOI] [PubMed] [Google Scholar]
- 11. Siontis GC, Juni P, Pilgrim T, Stortecky S, Bullesfeld L, Meier B, Wenaweser P, Windecker S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta‐analysis. J Am Coll Cardiol. 2014;129–140. [DOI] [PubMed] [Google Scholar]
- 12. Sawaya FRM. PPM Implantation After TAVR; Expert Analysis. accorg. 2019. Available at: https://www.acc.org/latest-in-cardiology/articles/2019/09/16/09/29/ppm-implantation-after-tavr. Accessed March 10, 2020.
- 13. Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alkhouli M, Alqahtani F, Ziada KM, Aljohani S, Holmes DR, Mathew V. Contemporary trends in the management of aortic stenosis in the USA. Eur Heart J. 2020;921–928. [DOI] [PubMed] [Google Scholar]
- 16. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;1690–1691. [DOI] [PubMed] [Google Scholar]
- 17. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;702–706. [DOI] [PubMed] [Google Scholar]
- 18. Urena M, Rodes‐Cabau J. Conduction abnormalities: the true Achilles' heel of transcatheter aortic valve replacement? JACC Cardiovasc Interv. 2016;2217–2219. [DOI] [PubMed] [Google Scholar]
- 19. van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new‐generation devices: a systematic review. Eur Heart J. 2018;2003–2013. [DOI] [PubMed] [Google Scholar]
- 20. Chamandi C, Barbanti M, Munoz‐Garcia A, Latib A, Nombela‐Franco L, Gutierrez‐Ibanez E, Veiga‐Fernandez G, Cheema AN, Cruz‐Gonzalez I, Serra V, et al. Long‐Term outcomes in patients with new‐onset persistent left bundle branch block following TAVR. JACC Cardiovasc Interv. 2019;1175–1184. [DOI] [PubMed] [Google Scholar]
- 21. Chamandi C, Barbanti M, Munoz‐Garcia A, Latib A, Nombela‐Franco L, Gutierrez‐Ibanez E, Veiga‐Fernandez G, Cheema AN, Cruz‐Gonzalez I, Serra V, et al. Long‐Term outcomes in patients with new permanent pacemaker implantation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;301–310. [DOI] [PubMed] [Google Scholar]
- 22. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez‐Gabella T, Philippon F, Rodes‐Cabau J. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;1049–1069. [DOI] [PubMed] [Google Scholar]
- 23. Rampat R, Khawaja MZ, Hilling‐Smith R, Byrne J, MacCarthy P, Blackman DJ, Krishnamurthy A, Gunarathne A, Kovac J, Banning A, et al. Conduction abnormalities and permanent pacemaker implantation after transcatheter aortic valve replacement using the repositionable LOTUS Device: the United Kingdom experience. JACC Cardiovasc Interv. 2017;1247–1253. [DOI] [PubMed] [Google Scholar]
- 24. Regueiro A, Abdul‐Jawad Altisent O, Del Trigo M, Campelo‐Parada F, Puri R, Urena M, Philippon F, Rodes‐Cabau J. Impact of new‐onset left bundle branch block and periprocedural permanent pacemaker implantation on clinical outcomes in patients undergoing transcatheter aortic valve replacement: a systematic review and meta‐analysis. Circ Cardiovasc Interv. 2016;e003635 DOI: 10.1161/CIRCINTERVENTIONS.115.003635. [DOI] [PubMed] [Google Scholar]
- 25.(www.accessdata.fda.gov) Food and Drug Administration. Medtronic CoreValve Transcatheter Aortic Valve. 2014.
- 26. Mauri V, Reimann A, Stern D, Scherner M, Kuhn E, Rudolph V, Rosenkranz S, Eghbalzadeh K, Friedrichs K, Wahlers T, et al. Predictors of permanent pacemaker implantation after transcatheter aortic valve replacement with the SAPIEN 3. JACC Cardiovasc Interv. 2016;2200–2209. [DOI] [PubMed] [Google Scholar]
- 27. Schymik G, Wendler O, Hengstenberg C, Ohlmann P, Gilard M, Digne F, Souteyrand G, Letocart V, van Belle E, Bramlage P, et al. Outcomes of transfemoral balloon expandable transcatheter aortic valve implantation: Comparison of two subsequent valve generations. Catheter Cardiovasc Interv. 2019. Dec 3 [epub ahead of print]. DOI: 10.1002/ccd.28621. [DOI] [PubMed] [Google Scholar]
- 28. Hellhammer K, Piayda K, Afzal S, Kleinebrecht L, Makosch M, Hennig I, Quast C, Jung C, Polzin A, Westenfeld R, et al. The latest evolution of the medtronic CoreValve system in the era of transcatheter aortic valve replacement: matched comparison of the Evolut PRO and Evolut R. JACC Cardiovasc Interv. 2018;2314–2322. [DOI] [PubMed] [Google Scholar]
- 29. Landes U, Bental T, Barsheshet A, Assali A, Vaknin Assa H, Levi A, Orvin K, Kornowski R. Comparative matched outcome of evolut‐r vs corevalve transcatheter aortic valve implantation. J Invasive Cardiol. 2017;69–74. [PubMed] [Google Scholar]
- 30. De Torres‐Alba F, Kaleschke G, Diller GP, Vormbrock J, Orwat S, Radke R, Reinke F, Fischer D, Reinecke H, Baumgartner H. Changes in the pacemaker rate after transition from Edwards SAPIEN XT to SAPIEN 3 transcatheter aortic valve implantation: the critical role of valve implantation height. JACC Cardiovasc Interv. 2016;805–813. [DOI] [PubMed] [Google Scholar]
- 31. Reardon MJ, Feldman TE, Meduri CU, Makkar RR, O'Hair D, Linke A, Kereiakes DJ, Waksman R, Babliaros V, Stoler RC, et al. Two‐year outcomes after transcatheter aortic valve replacement with mechanical vs self‐expanding valves: the REPRISE III randomized clinical trial. JAMA Cardiol. 2019;223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mangieri A, Lanzillo G, Bertoldi L, Jabbour RJ, Regazzoli D, Ancona MB, Tanaka A, Mitomo S, Garducci S, Montalto C, et al. Predictors of advanced conduction disturbances requiring a late (>/=48 H) permanent pacemaker following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;1519–1526. [DOI] [PubMed] [Google Scholar]
- 33. Tian Y, Padmanabhan D, McLeod CJ, Zhang P, Xiao P, Sandhu GS, Greason KL, Gulati R, Nkomo VT, Rihal CS, et al. Utility of 30‐day continuous ambulatory monitoring to identify patients with delayed occurrence of atrioventricular block after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2019;e007635 DOI: 10.1161/CIRCINTERVENTIONS.118.007635. [DOI] [PubMed] [Google Scholar]
- 34. Sandhu A, Tzou W, Ream K, Valle J, Tompkins C, Nguyen DT, Sauer WH, Carroll JD, Messenger J, Aleong RG. Heart block after discharge in patients undergoing TAVR with latest‐generation valves. J Am Coll Cardiol. 2018;577–578. [DOI] [PubMed] [Google Scholar]
- 35. Meduri CU, Kereiakes DJ, Rajagopal V, Makkar RR, O'Hair D, Linke A, Waksman R, Babliaros V, Stoler RC, Mishkel GJ, et al. Pacemaker implantation and dependency after transcatheter aortic valve replacement in the REPRISE III trial. J Am Heart Assoc. 2019;e012594 DOI: 10.1161/JAHA.119.012594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Costa G, Zappulla P, Barbanti M, Cirasa A, Todaro D, Rapisarda G, Picci A, Platania F, Tosto A, Di Grazia A, et al. Pacemaker dependency after transcatheter aortic valve implantation: incidence, predictors and long‐term outcomes. EuroIntervention. 2019;875–883. [DOI] [PubMed] [Google Scholar]
- 37. Fujita B, Schmidt T, Bleiziffer S, Bauer T, Beckmann A, Bekeredjian R, Mollmann H, Walther T, Landwehr S, Hamm C, et al. Impact of new pacemaker implantation following surgical and transcatheter aortic valve replacement on 1‐year outcome. Eur J Cardiothorac Surg. 2020;151–159. [DOI] [PubMed] [Google Scholar]
- 38. Aljabbary T, Qiu F, Masih S, Fang J, Elbaz‐Greener G, Austin PC, Rodes‐Cabau J, Ko DT, Singh S, Wijeysundera HC. Association of clinical and economic outcomes with permanent pacemaker implantation after transcatheter aortic valve replacement. JAMA Netw Open. 2018;e180088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas PS, El‐Chami MF, Herrmann HC, Mack M, Makkar RR, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv. 2015;60–69. [DOI] [PubMed] [Google Scholar]
- 40. Greason KL, Lahr BD, Stulak JM, Cha YM, Rea RF, Schaff HV, Dearani JA. Long‐Term mortality effect of early pacemaker implantation after surgical aortic valve replacement. Ann Thorac Surg. 2017;1259–1264. [DOI] [PubMed] [Google Scholar]
- 41. Patel N, Doshi R, Kalra R, Bajaj NS, Arora G, Arora P. Costs of transcatheter aortic valve replacement: implications of proposed medicare cuts. JACC Cardiovasc Interv. 2018;610–612. [DOI] [PubMed] [Google Scholar]
- 42. Husser O, Pellegrini C, Kim WK, Holzamer A, Pilgrim T, Toggweiler S, Schafer U, Blumenstein J, Deuschl F, Rheude T, et al. Transcatheter valve SELECTion in Patients with right bundle branch block and impact on pacemaker implantations. JACC Cardiovasc Interv. 2019;1781–1793. [DOI] [PubMed] [Google Scholar]
- 43. Osman M, Ghaffar YA, Saleem M, Kheiri B, Osman K, Munir MB, Alkhouli M. Meta‐analysis comparing transcatheter aortic valve implantation with balloon versus self‐expandable valves. Am J Cardiol. 2019;1252–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


