Abstract
Background
We compared different methods of estimated glomerular filtration rate (eGFR) and their association with cardiovascular death and major bleeding in 14 980 patients with atrial fibrillation in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial.
Methods and Results
eGFR was calculated using equations based on creatinine (Cockcroft‐Gault, Modification of Diet in Renal Disease, and Chronic Kidney Disease Epidemiology Collaboration [CKD‐EPI]) and/or cystatin C (CKD‐EPICysC and CKD‐EPICysC+Creatinine). These 5 eGFR equations, as well as the individual variables that are used in these equations, were assessed for correlation and discriminatory ability for cardiovascular death and major bleeding. The median age was 70.0 years, and 35.6% were women. The median eGFR was highest with Cockcroft‐Gault (74.1 mL/min) and CKD‐EPICysC (74.2 mL/min), and lowest with Modification of Diet in Renal Disease (66.5 mL/min). Correlation between methods ranged from 0.49 (Cockroft‐Gault and CKD‐EPICysC) to 0.99 (Modification of Diet in Renal Disease and CKD‐EPI). Among the eGFR equations, those based on cystatin C yielded the highest C indices for cardiovascular death and major bleeding: 0.628 (CKD‐EPICysC) and 0.612 (CKD‐EPICysC+Creatinine), respectively. A model based on the variables within the different eGFR equations (age, sex, weight, creatinine, and cystatin C) yielded the highest discriminatory value for both outcomes, with a C index of 0.673 and 0.656, respectively.
Conclusions
In patients with atrial fibrillation on anticoagulation, correlation between eGFR calculated using different methods varied substantially. Cystatin C–based eGFRs seem to provide the most robust information for predicting death and bleeding. A model based on the individual variables within the eGFR equations, however, provided the highest discriminatory value. Our findings may help refine risk stratification in patients with atrial fibrillation and define how renal function should be determined in future atrial fibrillation studies.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00412984.
Keywords: atrial fibrillation, bleeding, outcome, renal function
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
- CKD‐EPI
Chronic Kidney Disease Epidemiology Collaboration
- CysC
cystatin C
- GFR
glomerular filtration rate
- eGFR
estimated glomerular filtration rate
- IQR
interquartile range
- ISTH
International Society of Thrombosis and Haemostasis
- MDRD
Modification of Diet in Renal Disease
Clinical Perspective
What Is New?
This study is the first to systematically evaluate different methods of estimating renal function in a large cohort of patients with atrial fibrillation, describe their correlation, and determine their associations with cardiovascular death and major bleeding.
What Are the Clinical Implications?
The results show that the correlation between estimated glomerular filtration rate methods varied substantially.
Cystatin C–based estimated glomerular filtration rate provided the most robust discrimination of the risk of death and bleeding over a broad range of renal function.
The findings may help to refine risk stratification in patients with atrial fibrillation.
Impaired renal function is associated with increased risk of cardiovascular events and bleeding in patients with atrial fibrillation (AF). 1 , 2 , 3 , 4 Serum creatinine is often used to estimate glomerular filtration rate (GFR) since a more accurate measurement requires urinary or plasma clearance of exogenous markers, which is often not practical in routine clinical use. Several creatinine‐based methods are available to estimate renal function, such as Cockcroft‐Gault, Modification of Diet in Renal Disease (MDRD), and Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI). While used widely in practice to adjust doses of renally eliminated drugs, Cockcroft‐Gault was developed using a modest number of patients that did not include women, 5 and estimates of renal function have been shown to predict outcomes but with little evidence about comparative predictive value. Some limitations exist with creatinine that may cause inaccurate estimations dependent on factors such as age, sex, ethnicity, muscle mass, and dietary intake. 6 To overcome these issues, CysC (cystatin C), a small protein proposed to be a more reliable marker of renal function than serum creatinine, may be used to estimate GFR. More recently, a new method for estimated GFR (eGFR) was presented in an equation using information from both serum creatinine and CysC simultaneously. 7 , 8 The combined creatinine‐CysC equation performed better than equations based on either of these markers for estimation of renal function as well as risk stratification. 7 , 9 However, the different eGFR equations have not been systematically evaluated in patients with AF on oral anticoagulation. Our aim in this ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) substudy was to evaluate the different eGFR equations, both the traditional creatinine‐based equations as well as the newer CysC eGFR equations, for potential differences in distribution, agreement, and prognostic value concerning risk of cardiovascular death and major bleeding. The evaluation of risk also included a model based on the individual variables included in the eGFR equations.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Population
Ethics committee approval for the ARISTOTLE trial (Clinicaltrials.gov identifier NCT00412984) was obtained for all investigational sites, and all patients provided written informed consent. The study complies with the Declaration of Helsinki. To be eligible in the ARISTOTLE trial, patients had to have AF or flutter at enrollment or at least 2 episodes of AF or flutter documented by ECG at least 2 weeks apart in the 12 months before enrollment. In addition, at least 1 of the following risk factors for stroke was required: age ≥75 years; prior stroke, transient ischemic attack, or systemic embolism; symptomatic heart failure within 3 months or left ventricular ejection fraction of no more than 40%; diabetes mellitus; and hypertension requiring pharmacologic treatment. Major exclusion criteria included AF attributable to a reversible cause, moderate or severe mitral stenosis, conditions other than AF that required anticoagulation such as prosthetic heart valve, stroke within 7 days, need for aspirin >165 mg/d or both aspirin and clopidogrel, and severe renal insufficiency (serum creatinine >2.5 mg/dL [221 µmol/L] or calculated creatinine clearance <25 mL/min by Cockroft‐Gault). 10 The ARISTOTLE trial was conducted at 1034 clinical sites in 39 countries between December 2006 and April 2010. A total of 18 201 patients were included in the ARISTOTLE trial; of these, 14 980 participated in the biomarker program, of whom 14 884 had CysC measurements available.
Trial Design and Outcome Measures
The design of the ARISTOTLE trial has been published. 10 In brief, eligible patients were randomly assigned to receive apixaban or dose‐adjusted warfarin using a double‐blind, double‐dummy design. Warfarin (or matching placebo) was provided as 2‐mg tablets adjusted to achieve a target international normalized ratio of 2.0–3.0. International normalized ratios were monitored using a blinded, encrypted point‐of‐care international normalized ratio device, and an algorithm was provided to guide warfarin dose adjustment.
The outcomes in this substudy, cardiovascular death and major bleeding, were both among the prespecified efficacy and safety end points in the ARISTOTLE trial. Major bleeding was the primary safety outcome and defined according to the International Society of Thrombosis and Haemostasis (ISTH); acute or subacute clinically overt bleeding accompanied by ≥1 of the following: (1) a decrease in hemoglobin level of ≥2 g/dL over a 24‐hour period; (2) a transfusion of ≥2 U of packed red blood cells; and/or (3) bleeding that is fatal or occurs in at least 1 of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intraarticular, intramuscular with compartment syndrome, or retroperitoneal. 9 A blinded clinical events committee adjudicated all efficacy and safety outcomes according to prespecified criteria.
Patient and Public Involvement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting of our research.
Laboratory Methods
At the time of randomization, before initiation of study treatment, a venous EDTA blood sample was drawn for determination of creatinine and CysC levels. The blood was centrifuged, and thereafter plasma was immediately frozen at −20°C or colder. Aliquots were stored at −70°C to allow batch analysis. Creatinine measurements were performed in a core laboratory using a Roche Modular analyzer with a kinetic colorimetric compensated Jaffe assay. CysC was centrally analyzed in the Uppsala Clinical Research Center laboratory, Sweden, with the Architect system ci8200 (Abbott Laboratories) using the particle‐enhanced turbidimetric immunoassay from Gentian (Gentian). The lower limit of detection is 0.05 mg/L according to the manufacturer. The total analytical precision of the method is 1.09% at 0.85 mg/L and 1.03% at 3.06 mg/L. 11 The upper reference level, defined as the 97.5th percentile value in an apparently healthy population, is 1.21 mg/L for those aged >65 years. 12
GFR Estimation
The following equations were used to calculate creatinine‐based eGFR:
Cockcroft‐Gault:
MDRD:
CKD‐EPI:
α=−0.329 (female) or −0.411 (males); K=0.7 (female) or 0.9 (males).
The following equation was used to calculate CysC‐based eGFR:
CKD‐EPI CysC:
The following equation was used to calculate eGFR using both creatinine and CysC eGFR:
CKD‐EPI CysC+Creatinine:
α=−0.248 (female) or −0.207 (males); K=0.7 (female) or 0.9 (males).
Statistical Analysis
Marginal and bivariate sampling distributions of the eGFRs calculated by the different methods are illustrated graphically using empirical cumulative density function plots and scatterplots, respectively. Agreement between the different eGFR methods was assessed using Bland‐Altman plots. The association between each eGFR method and cardiovascular death and major bleeding, respectively, was evaluated using Cox regression models including natural log‐transformed eGFR as a restricted cubic spline with 5 knots placed at the 5th, 27.5th, 50th, 72.5th, and 95th sample percentiles. The associations were presented graphically and the discriminative ability of each eGFR method was assessed using Harrell C index. Patients were followed until the respective event occurred or, if the event did not occur, were censored at the end of the study or, for major bleeding, at death. Since the purpose of the study was to compare the different eGFR equations with each other and with a regression model including the same variables as the eGFR equations include, no other variables were considered as confounders.
In addition, a simple regression model was constructed that included a linear combination of the variables used in the eGFR equations (age, sex, weight, and CysC and/or creatinine). CysC and creatinine were log‐transformed and represented as restricted cubic splines in the models. The discriminative ability of these models was evaluated by Harrell C index. All analyses were performed using R version 3.5 (The R Foundation).
Results
Patient Characteristics and eGFR by Different Estimates
The median age of patients was 70.0 years, and 35.6% were women (Table S1). Median eGFR with creatinine‐based equations were: Cockcroft‐Gault 74.1 (interquartile range [IQR], 56.8–95.3), MDRD 66.5 mL/min (IQR, 55.2–78.6), and CKD‐EPICreatinine 68.5 mL/min (IQR, 55.9–82.0). With CysC‐based equations, the eGFRs were: CKD‐EPICysC 74.2 mL/min (IQR, 56.7–96.0), and CKD‐EPICysC+Creatinine 72.6 mL/min (IQR, 57.9–87.4). The distributions of eGFR according to each method are shown in Figure 1, and the proportion of patients with a GFR <60 mL/min in Table S2.
Figure 1. Distributions of estimated renal function by different equations.

Empirical cumulative distribution function plot of estimated renal function according to 5 different equations. CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; eGFR, estimated glomerular filtration rate; and MDRD, Modification of Diet in Renal Disease.
The agreement between the different eGFR equations were assessed using scatterplots and Spearman correlation (Figure 2), and Bland‐Altman plots and mean differences (Figure 3). The correlation was highest between MDRD and CKD‐EPICreatinine, and between the 2 CysC‐based eGFR equations, CKD‐EPICysC and CKD‐EPICysC+Creatinine, with correlation coefficients of 0.99 and 0.94, respectively. However, a visual inspection of the scatterplot of MDRD and CKD‐EPICreatinine, showed that the high correlation was particularly valid at lower eGFR levels, as to a persistent disagreement was seen at higher renal function values as MDRD consistently tended to overestimate GFR as compared with the CKD‐EPICreatinine equation. On the contrary, for the CysC methods, CKD‐EPICysC and CKD‐EPICysC+Creatinine agreement was continual irrespective of eGFR values. The poorest correlation was seen between Cockcroft‐Gault and the CysC‐based CKD‐EPICysC equation, with a correlation coefficient of 0.49. Figure S1 shows how the different eGFR methods were affected by sex in different age groups. CysC‐based eGFR equations showed less variation in comparison with the creatinine‐based equations (Figure S1).
Figure 2. Correlation between estimated glomerular filtration rate (eGFR) methods.
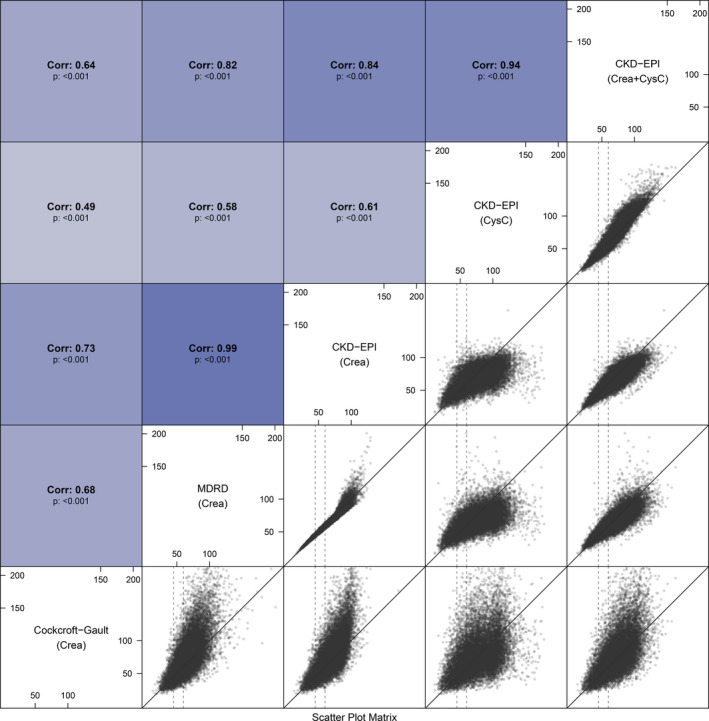
Above‐diagonal panels show the Spearman correlation coefficients between pairwise eGFR methods. Below‐diagonal panels show scatterplots of pairwise eGFR methods––the solid diagonal line is the 1:1 relationship. The dashed vertical lines show eGFR of 45 and 60 mL/min. CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; and MDRD, Modification of Diet in Renal Disease.
Figure 3. Agreement between estimated glomerular filtration rate (eGFR) methods, mean differences, and Bland‐Altman plots.
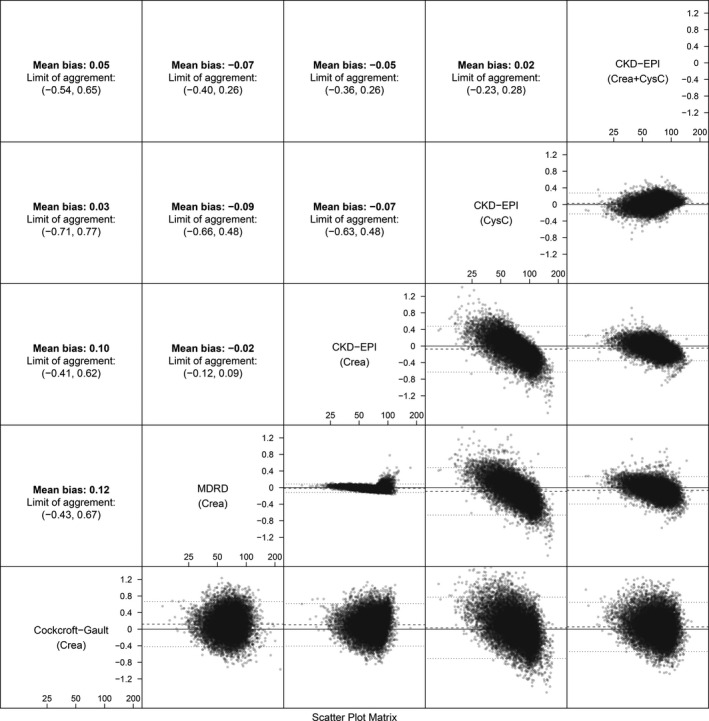
Above‐diagonal panels show the mean difference and the limit of agreement (±1.96 SD). Below‐diagonal panels show Bland‐Altman plots of the difference vs the mean of pairwise methods. CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; and MDRD, Modification of Diet in Renal Disease.
Different Estimates of Renal Function and Outcomes
During follow‐up, 547 events of cardiovascular death occurred and 674 events of major bleeding. The associations between continuous eGFR estimates and outcomes are shown in Figure 4. For all eGFR methods, compared with an eGFR reference value of 60 mL/min, the relative risk of cardiovascular death and major bleeding increased with decreasing eGFR. The relative risk of cardiovascular death decreased most as renal function improved according to the CysC‐based equations, using CysC alone, or in combination with creatinine. The results were similar for major bleeding with the addition of the CKD‐EPI equation based solely on creatinine. The distribution for each eGFR method is shown in the bottom of each panel in Figure 4.
Figure 4. Associations between continuous estimated glomerular filtration rate (eGFR) estimates and cardiovascular death (left panel) and major bleeding (right panel).

The reference point for each eGFR method is 60 mL/min, the relative hazard is shown on the y axis according to the different values of renal function on the x axis. Each line represents a different method of eGFR. The bottom of the figure shows the distribution for each eGFR method. CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; and MDRD, Modification of Diet in Renal Disease.
The prognostic value of each eGFR method and the simple regression models are presented in the Table. Among the eGFR equations, for the outcome of cardiovascular death, the CysC‐based method yielded the highest C index, 0.628 (CKD‐EPICysC), followed by the equations based on both biomarkers or on only creatinine (Table). For major bleeding, the eGFR equations based on the combination of CysC and creatinine, and the Cockcroft‐Gault method, provided the highest C indices, 0.612 for both (CKD‐EPICysC+Creatinine and Cockcroft‐Gault, respectively). The simple regression model including only the 2 biomarkers, CysC and creatinine, performed better than any of the eGFR methods for both outcomes. The model including only CysC performed better than all eGFR methods for cardiovascular death. The regression model based on the actual variables within the equations used to estimate eGFR (age, sex, weight, CysC and/or creatinine) consistently provided the best discriminatory value for cardiovascular death and for major bleeding (Table). Last, analyses adjusted for age, sex, and weight showed that the CysC‐based eGFR equations provided a higher prognostic value than eGFR equations using creatinine (Table).
Table 1.
Discriminative Ability of Each eGFR Method, Biomarker, and Regression Models, for Cardiovascular Death and Major Bleeding
| Cardiovascular Death | Major Bleeding | |||
|---|---|---|---|---|
| C Index | 95% CI | C Index | 95% CI | |
| eGFR method | ||||
| Cockcroft‐Gault | 0.608 | 0.582–0.634 | 0.612 | 0.589–0.635 |
| MDRD | 0.575 | 0.550–0.600 | 0.575 | 0.551–0.600 |
| CKD‐EPICreatinine | 0.579 | 0.553–0.605 | 0.592 | 0.568–0.616 |
| CKD‐EPICysC | 0.628 | 0.603–0.653 | 0.611 | 0.588–0.634 |
| CKD‐EPICysC+Creatinine | 0.615 | 0.590–0.640 | 0.612 | 0.589–0.636 |
| Biomarkers | ||||
| Creatinine | 0.584 | 0.558–0.611 | 0.582 | 0.558–0.605 |
| CysC | 0.631 | 0.606–0.656 | 0.608 | 0.585–0.631 |
| Creatinine+CysC | 0.637 | 0.612–0.661 | 0.619 | 0.597–0.642 |
| Regression model | ||||
| Age+sex+weight | 0.618 | 0.593–0.642 | 0.627 | 0.605–0.648 |
| Regression model and biomarkers | ||||
| Age+sex+weight+creatinine | 0.633 | 0.609–0.658 | 0.645 | 0.623–0.667 |
| Age+sex+weight+CysC | 0.667 | 0.643–0.692 | 0.649 | 0.627–0.671 |
| Age+sex+weight+creatinine+CysC | 0.673 | 0.649–0.696 | 0.656 | 0.634–0.678 |
| Regression model and eGFR | ||||
| Age+sex+weight+Cockcroft‐Gault | 0.638 | 0.614–0.662 | 0.640 | 0.618–0.662 |
| Age+sex+weight+MDRD | 0.633 | 0.609–0.658 | 0.642 | 0.620–0.664 |
| Age+sex+weight+CKD‐EPICreatinine | 0.637 | 0.613–0.662 | 0.642 | 0.621–0.664 |
| Age+sex+weight+CKD‐EPICysC | 0.666 | 0.642–0.690 | 0.648 | 0.626–0.670 |
| Age+sex+weight+CKD‐EPICysC+Creatinine | 0.657 | 0.632–0.681 | 0.650 | 0.628–0.672 |
CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CysC, Cystatin C; eGFR, estimated glomerular filtration rate; and MDRD, Modification of Diet in Renal Disease.
Discussion
In this study, we systematically evaluated several methods of calculating eGFR in a large cohort of patients with AF on oral anticoagulation concerning their distribution, correlation, and the association of the different formulas with death and major bleeding. The results show large differences in the correlation between different eGFR methods as well as their discriminatory values. Overall, the CysC‐based estimates of eGFR seem to have a higher correlation and provide greater prognostic value concerning cardiovascular death and major bleeding.
In clinical practice, evaluation of renal function is important regarding correct drug dosing as well as for risk assessment. 13 Several prior publications have investigated the performance of each eGFR method in regards to how precisely they correlate with actual GFR measured with invasive methods. Equations based on CysC provide advantages in comparison with those based on creatinine. 7 , 9 More recently, a newer equation for eGFR was proposed, using both CysC and creatinine simultaneously in the equation. The combined creatinine‐CysC method seems to reflect actual GFR even more accurately, and provides a better risk stratification in large cohorts of general populations and patients with chronic kidney disease. 7 , 9 The results in the present study expand upon these findings to a large cohort of patients with AF on oral anticoagulation. Importantly, it also adds data for the outcome of major bleeding. This study also showed that the correlation between the different eGFR equation were poor at times. Since we do not have data on the actual GFR, no conclusions can be drawn in regards to which equation reflects renal function more accurately in this material. However, from a risk perspective, the current findings support the concept that eGFR methods that incorporate CysC provide the best balance concerning risk of cardiovascular death and major bleeding.
CysC has been proposed to be a more reliable marker of renal function than serum creatinine because it is synthetized at a constant rate in all nucleated cells and it is a small protein, freely filtrated by the glomerulus, without returning to the blood flow. 8 , 14 , 15 CysC is also less affected by age and sex, 7 which was also affirmed in this large cohort of patients with AF. Together, these advantages might provide an explanation for the current results. Another important finding is that a model based on the actual variables within the equations used to estimate GFR (age, sex, weight, creatinine/CysC) provides better discrimination for cardiovascular death and for major bleeding than the different eGFR methods. This might not be fully unexpected, since in such a model, individual regression parameters are estimated for each variable's association with these outcomes, as opposed to the eGFR equations, in which the variables are fixed to reflect actual GFR. From a methodologic viewpoint, this fact carries importance as the results support that it is preferable to use the actual variables within the eGFR equations (age, sex, weight, creatinine/CysC), as variables in multivariable regression models, as opposed to using a calculated eGFR based on the same variables, to provide the most robust clinical risk adjustment, and to avoid collinearity. Since Cockcroft‐Gault has been the method used to generate evidence regarding dose adjustment of oral anticoagulants such as rivaroxaban and edoxaban, our findings should not be used to change that approach.
Limitations
The ARISTOTLE trial excluded patients with severely reduced renal function (<25 mL/min). Also, since data were not available on actual GFR, no conclusions can be drawn in regards to which eGFR method most accurately represents true GFR. Since there was a lower frequency of patients with eGFR >105 mL/min according to the MDRD and creatinine‐based CKD‐EPI equation, the hazard ratios in the upper spectrum of renal function with these 2 methods may need to be interpreted with caution.
Conclusions
In patients with AF on oral anticoagulation, correlation between eGFR methods varied substantially. CysC‐based eGFR provided the most robust discrimination of the risk of death and bleeding over a broad range of renal function. However, a model based on the individual variables within the eGFR equations (age, sex, weight, creatinine, CysC) yielded the highest discriminatory value. Our findings may help to refine risk stratification in patients with AF and define how renal function should be determined in future AF studies.
Sources of Funding
This work was supported by and the ARISTOTLE trial was funded by Bristol‐Myers Squibb and Pfizer. and coordinated by the Duke Clinical Research Institute and Uppsala Clinical Research Center. The analyses were supported by Bristol‐Myers Squibb and Pfizer. The funders were given the opportunity to review and comment on the final version of the article.
Disclosures
Z.H. reports lecture fees from Boehringer Ingelheim, Bristol‐Myers Squibb, Pfizer, and Roche Diagnostics; consulting fees from Boehringer Ingelheim, Bristol‐Myers Squibb, Meda, Merck Sharp & Dohme, Pfizer, and Roche Diagnostics; and research grants from the Swedish Society for Medical Research [S17‐0133] and the Swedish Heart‐Lung Foundation [20170718]. C.B.G. reports research grants and consulting/speaker fees from Boehringer Ingelheim, Bristol‐Myers Squibb, Janssen Pharmaceuticals, Pfizer, AstraZeneca, and Novartis; research grants from Daichii‐Sankyo, AKROS, Apple, GlaxoSmithKline, and the US Food & Drug Administration; and consulting/speaker fees from Bayer Corp, Boston Scientific Corp, Abbvie, Espero BioPharma, Medscape, Merck, National Institutes of Health, NovoNordisk, Roche Diagnostics, Rho Diagnostics, Sirtex, and Verseon. S.H.H. reports research grants, consulting fees, and lecture fees from Sanofi and St Jude Medical; consulting and lecture fees from Bristol Myers Squibb, Pfizer, Bayer Healthcare, Boehringer Ingelheim, Daichii‐Sankyo, and Medtronic; and consulting fees from Boston Scientific, Cardiome, Gilead, Johnson & Johnson, Portola, Servier, and Zoll. J.W. reports institutional research grants from Bristol‐Myers Squibb/Pfizer. J.L. reports institutional research grants from Bristol‐Myers Squibb/Pfizer. J.H.A. reports institutional research grants and consulting fees/honoraria from Bristol‐Myers Squibb and CSL Behring; institutional research grants from AstraZeneca, CryoLife, the US Food & Drug Administration, National Institutes of Health, Sanofi, VoluMetrix, and Boehringer Ingelheim; and consulting fees/honoraria from Pfizer, AbbVie Pharmaceuticals, NovoNordisk, Portola Pharmaceuticals, Quantum Genetics, Teikoku Pharmaceuticals, VA Cooperative Studies Program, and Zafgen. M.K. has nothing to report. A.P. reports grants and personal fees from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi. J.L.L. reports personal fees from Boehringer Ingelheim; grants from Bayer, GlaxoSmithKline; grants and personal fees from Novartis, Servier, Menarini, Pfizer and Sanofi. R.D.L. reports institutional research grants and consulting fees from Bristol‐Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and consulting fees from Amgen, Bayer, and Boehringer Ingelheim. A.S. reports institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, GlaxoSmithKline, Roche Diagnostics; consulting fees from Olink Proteomics. L.W. reports institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, GlaxoSmithKline, Merck & Co, Roche Diagnostics; and consulting fees from Abbott.
Supporting information
Tables S1–S2
Figure S1
Acknowledgments
Editorial support was provided by Vendela Roos, PhD, Uppsala Clinical Research Center, Uppsala, Sweden.
(J Am Heart Assoc. 2020;9:e017155 DOI: 10.1161/JAHA.120.017155.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134:24–36. [DOI] [PubMed] [Google Scholar]
- 2. Fordyce CB, Hellkamp AS, Lokhnygina Y, Lindner SM, Piccini JP, Becker RC, Berkowitz SD, Breithardt G, Fox KA, Mahaffey KW, et al. On-treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134:37–47. [DOI] [PubMed] [Google Scholar]
- 3. Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (randomized evaluation of long-term anticoagulation therapy) trial analysis. Circulation. 2014;129:961–970. [DOI] [PubMed] [Google Scholar]
- 4. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez-Sendon J, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–2830. [DOI] [PubMed] [Google Scholar]
- 5. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 6. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. [DOI] [PubMed] [Google Scholar]
- 7. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 9. Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J, et al. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331–339. [DOI] [PubMed] [Google Scholar]
- 11. Flodin M, Jonsson AS, Hansson LO, Danielsson LA, Larsson A. Evaluation of Gentian cystatin C reagent on Abbott Ci8200 and calculation of glomerular filtration rate expressed in mL/min/1.73 m(2) from the cystatin C values in mg/L. Scand J Clin Lab Invest. 2007;67:560–567. [DOI] [PubMed] [Google Scholar]
- 12. Johnston N, Jernberg T, Lindahl B, Lindbäck J, Stridsberg M, Larsson A, Venge P, Wallentin L. Biochemical indicators of cardiac and renal function in a healthy elderly population. Clin Biochem. 2004;37:210–216. [DOI] [PubMed] [Google Scholar]
- 13. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 14. Abrahamson M, Olafsson I, Palsdottir A, Ulvsbäck M, Lundwall A, Jensson O, Grubb A. Structure and expression of the human cystatin C gene. Biochem J. 1990;268:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figure S1


