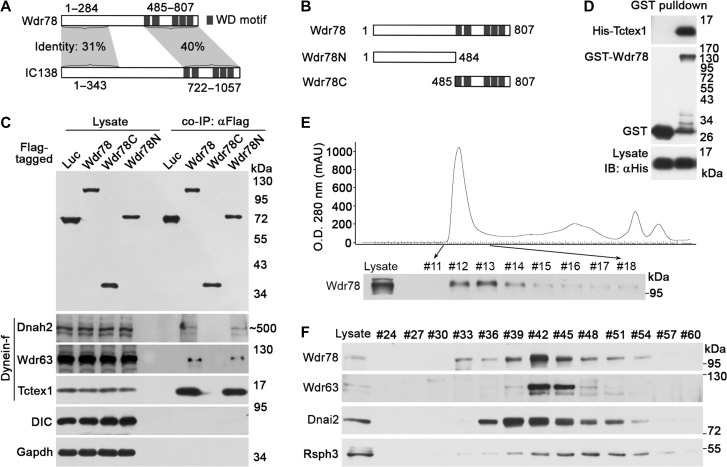
Figure 1.
Wdr78 is homologous to IC138 and associates with multiple subunits of Dynein-f. (A) A comparison between Wdr78 and IC138. The numbers indicate amino acid positions. (B) Schematic diagrams of Wdr78 and mutants. The numbers indicate amino acid positions. (C) Associations of Flag-Wdr78 with Dynein-f in co-IP. Flag-tagged Wdr78 and its mutants expressed in HEK293T cells were used to co-immunoprecipitate their associated proteins from mouse testis lysates. DIC and Gapdh served as negative controls. (D) Tctex1 directly bound to Wdr78 in vitro. Bacterial lysates expressing the indicated proteins were mixed and subjected to GST pull-down assays. (E and F) Endogenous Wdr78 co-fractionated with Dynein-f in sequential column chromatography. Mouse testis lysates were applied to a size-exclusion Superose 6 10/300 GL column. (E) Eluted fractions were collected and subjected to immunoblotting to detect Wdr78. The fractions #12–#14 were combined and applied to an anion-exchange Mono Q 4.6/100 PE column. The bound proteins were eluted using a linear salt gradient. (F) The indicated fractions were analyzed by immunoblotting.