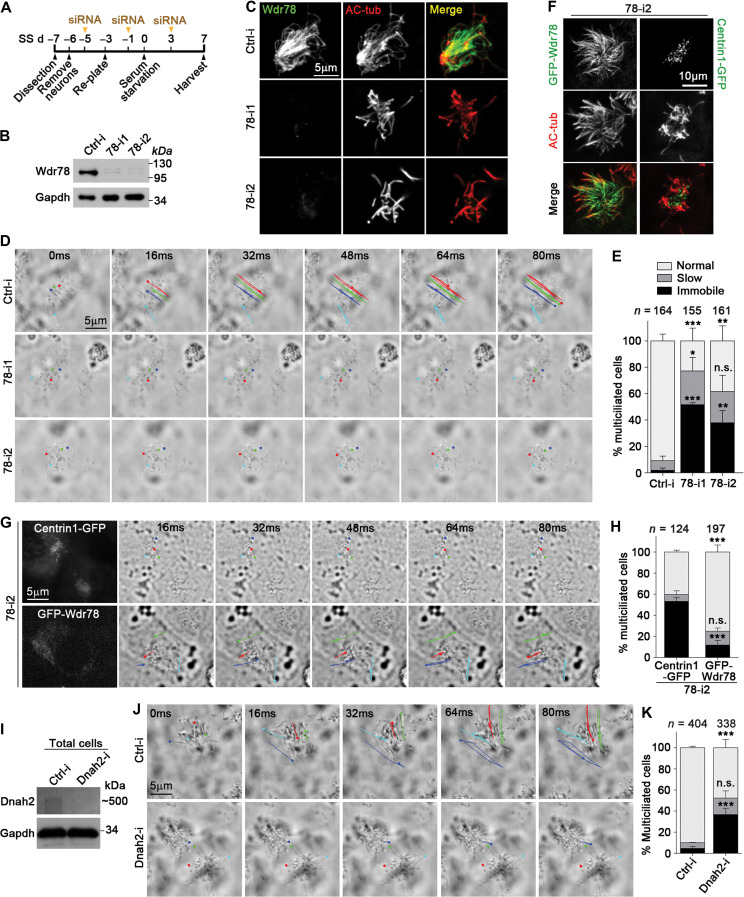
Figure 3.
Vertebrate Dynein-f is critical for ciliary beat. (A) Experimental scheme for RNAi in mEPCs. Glial cells from dissected mouse telencephalon tissues were induced to differentiate into multiciliated mEPCs through serum starvation at day 0 (SS d0). The cells were transfected three times with siRNA. (B and C) Efficient knockdown of Wdr78 by RNAi. Gapdh and AC-tub served as loading control and ciliary marker, respectively. (D and E) Depletion of Wdr78 paralyzed ependymal cilia. Ciliary movements were monitored by bright-field microscopy. (D) Trajectories of four cilia during the first 80 ms of imaging are shown for each mEPC. Please also refer to Supplementary Video S1. (F–H) GFP-Wdr78, but not Centrin1-GFP, restored ciliary motility in 78-i2-treated mEPCs. In addition to the transfections with 78-i2 in A, the cells were also infected with lentivirus at SS d–2 to express an siRNA-resistant GFP-Wdr78 or Centrin1-GFP. (F) The ciliary localization of GFP-Wdr78 was obvious in fixed mEPCs. (G) Due to the difficulty to clearly capture the fluorescent images of rapidly beating cilia, live GFP-positive cells were identified by the fluorescence in their cell bodies. Please refer to Supplementary Video S2. (I–K) Depletion of Dnah2 also caused immobile cilia in mEPCs. Please refer to Supplementary Video S3. Quantification results in E, H, and K were from three independent experiments and presented as mean ± SD. Cell numbers analyzed are listed over each histogram. Student’s t-test: n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001.