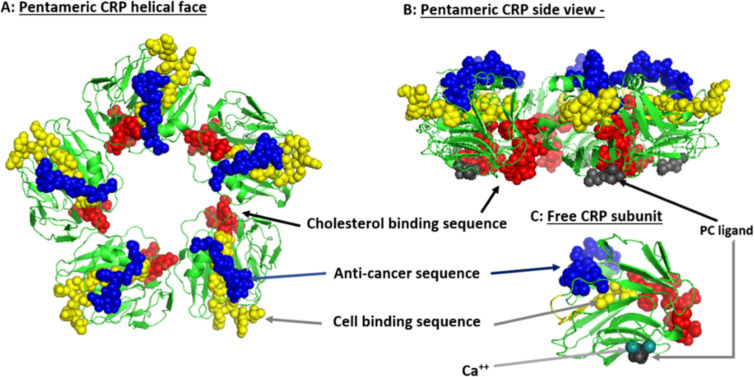
Figure 2.
Structural features of serum-soluble pentameric CRP. (A) illustrates the location and orientation peptide sequences in CRP reported to have cell-binding activity (shown in yellow and involving 27TKPLKAFTVCLH38) (105), anti-cancer activity (shown in blue and involving 176LGGPFSPNVL185) (106), cholesterol binding activity (shown in red and involving 35VCLHFYTELSSTR47), and which also controls CRP binding to apolipoprotein B, C1q, fibronectin, and collagen (107), in relationship to the phosphocholine (PC) binding face (PC groups shown in gray and involving residues L64, F66, and T76) and bound calcium ions (two per subunit, juxtaposed to each PC binding sites and involves residue E147) (PDB code: 1B09; PC and calcium residues as defined by Thompson et al. (108) and Shrive et al. (109), respectively). (B) illustrates the orientation of these same residues when the discoid protein is laid flat (i.e. side view). (C) shows the orientation of same sequences on the isolated pCRP subunit (note: the exact orientation of these residues on the conformationally changed mCRP subunit has not been determined). The PC ligand binds in a shallow binding pocket controlled by calcium ions, with all PC sites on one face of the flattened discoid structure. The cholesterol binding sequences are near the PC binding sites so that when pCRP binds membrane associated PC, the cholesterol binding sequence is brought into proximity with intra-membraneous cholesterol (in lipid rafts) contributing to the conversion of pCRP into mCRP. The cell binding and anti-cancer peptides are oriented on the opposite face of the discoid protein where they can interact with effector leukocytes and activated inflammatory responses.