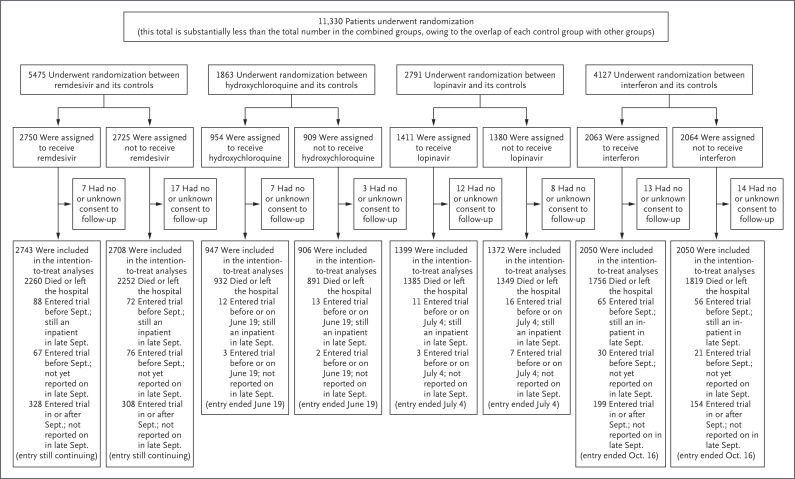
Figure 1. Information to October 4, 2020, on Trial Entry, Follow-up, and Intention-to-Treat Analyses.
After it was determined which treatments were locally available, random assignment (with equal probability) was between the local standard of care and the available treatments. After the exclusion of 64 of 11,330 patients (0.6%) who had provided either no or uncertain consent regarding follow-up, 11,266 remained in the intention-to-treat analyses. Each pairwise intention-to-treat analysis was between a particular trial drug and its control (i.e., patients who could have been assigned to a particular trial drug but were concurrently assigned to the same care without it). There is partial overlap of each control group with other groups.