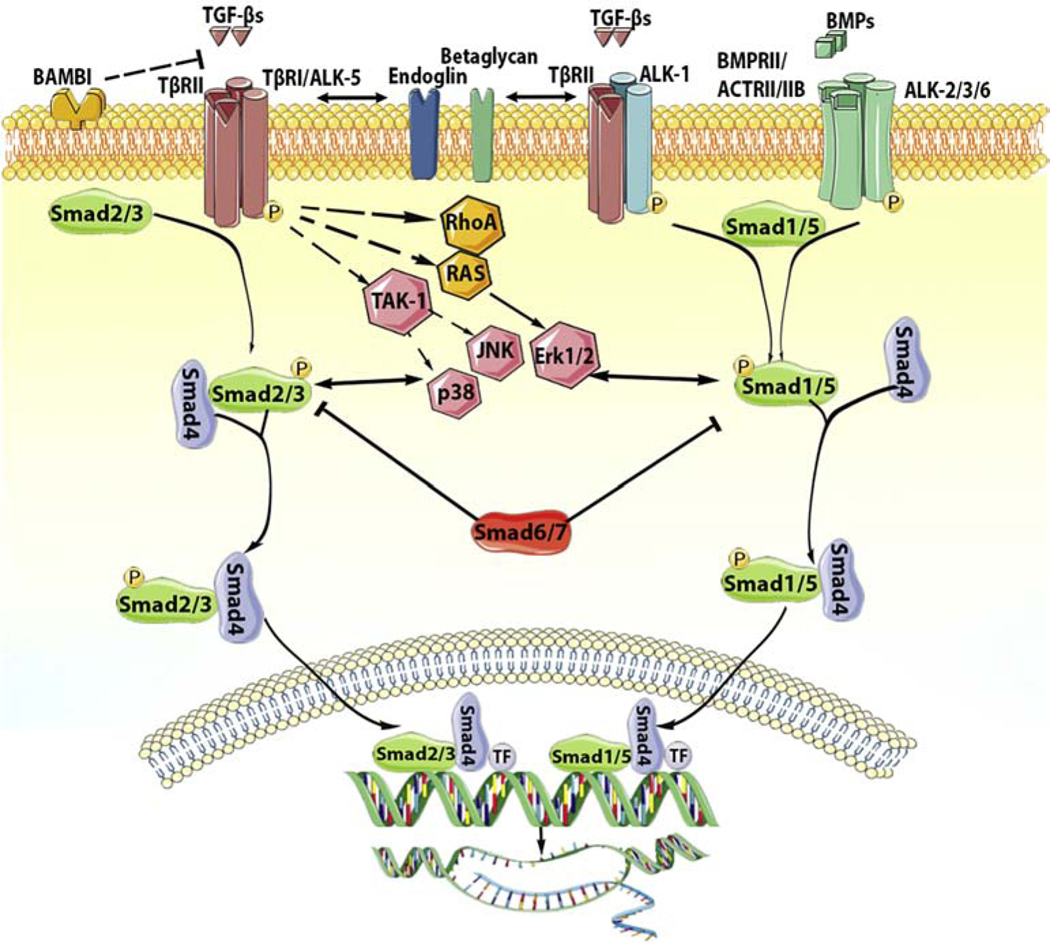
Figure 1: Signaling cascades activated by the TGF-β superfamily.
TGF-β superfamily ligands signal by binding to distinct combinations of two type II, and two type I receptors. TGF-βs bind TβRII inducing transphosphorylation of the type I receptor ALK5. Activated ALK5 phosphorylates the receptor-activated Smads (R-Smads) Smad2 and Smad3, which then form a heterotrimeric complex with the common Smad, Smad4, promoting the translocation of the Smad complex to the nucleus. Interactions between the Smad complex and transcriptional activators or repressors regulate transcription of target genes. In some cell types, TGF-βs may also act through another type I receptor, ALK1, stimulating Smad1/5/8 signaling. BMPs also bind to their specific type II receptors, subsequently activating type I receptors (ALK2, ALK3, ALK6) and phosphorylating Smad1/5. Smad1 and Smad5 then bind to Smad4 and translocate to the nucleus regulating transcription. In addition to the Smad-dependent signaling, TGF-β superfamily members may also signal through non-canonical Smad-independent cascades. The inhibitory Smads (Smad6 and Smad7) negatively regulate TGF-β superfamily cascades. Accessory receptors, such as endoglin and betaglycan, modulate TGF-β signaling. The cartoon was designed using Servier Medical Art (https://smart.servier.com/).