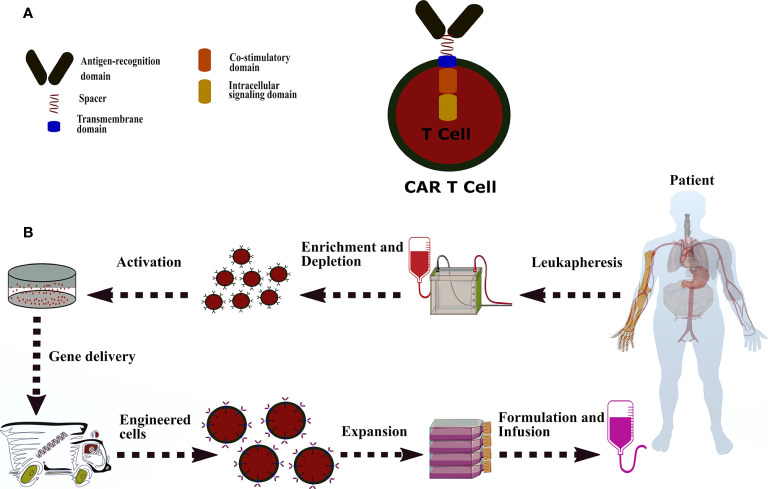
Figure 1.
A common CAR construction. (A): CAR is comprised of antigen-recognition domain (scFv), a hinge domain or spacer (CD28, CD8α, CD7, IgG4, and IgG1), a transmembrane domain (CD28, CD8α, CD7, CD4, CD3z, and OX40), a co-stimulatory domain (CD244, CD28, CD27, OX40, ICOS, and CD137), and a signaling domain (CD3z, DAP10, and DAP12). (B): The process of CAR-T manufacturing from peripheral blood mononuclear cells to genetically modified-T cells and administration. CAR-T therapy starts with accumulating the patient’s white blood cells by leukapheresis. The apheresis products are enriched or deleted for a specific cell subset and then activated by one of following methods, including interleukins (IL-2, IL-7, and IL-15), anti-CD3/CD28 antibody-coated magnetic beads, soluble CD3 antibody, artificial antigen-presenting cells, plate-bound antibodies, and adhesion molecules. The activated T cells are introduced with the CAR transgene through lentiviral or retroviral and non-viral methods (electroporation of naked DNA and plasmid-based transposon/transposase). Afterwards, the engineered-T cells undergo an expansion process in static culture bags or dynamic culture vessels or rotating bioreactors. Eventually, cell numbers are calculated based on the patient’s disease burden, weight, and another formulation. The CAR modified-T cells transfer to either a container for infusion purposes or cryopreserved for storage.