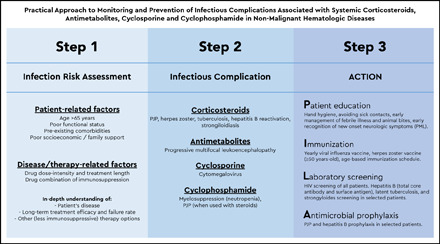
Visual Abstract
Abstract
Corticosteroids constitute a first-line therapy for adults and children suffering from nonmalignant immune-mediated hematologic diseases. However, high disease relapse rates during the tapering period or upon drug discontinuation result in long-term corticosteroid use that increases the risk of infection. This same concept applies to other immunosuppressive agents, such as antimetabolites, calcineurin inhibitors, and cyclophosphamide. Corticosteroids are associated with a length-of-treatment and dose-dependent risk for infection. Screening and antimicrobial prophylaxis against tuberculosis, hepatitis B, Strongyloides stercoralis, and Pneumocystis jirovecii pneumonia (PJP) might be indicated in patients who are scheduled to be on high-dose corticosteroids for >4 weeks (>30 mg of prednisone-equivalent dose [PEQ]) or in patients chronically treated (≥8 weeks of continuous or intermittent corticosteroid use) with moderate doses (≥15 to <30 mg PEQ). Antimetabolites (azathioprine, mycophenolate) increase the risk of progressive multifocal leukoencephalopathy (PML); however, other opportunistic infections and viral reactivation have also been reported. In case of new onset of neurological symptoms, PML needs to be considered, and an urgent neurology consultation should be obtained. Cyclophosphamide-induced myelosuppression can lead to serious infections related to neutropenia. PJP prophylaxis should be considered with combination therapy of cyclophosphamide and corticosteroids until a PEQ dose ≤ 5 mg/d is reached. Data on infectious risk when cyclosporine is used in patients with nonmalignant hematologic diseases are lacking. Discontinuation of any immunosuppressive agent during an episode of infection is recommended. In all patients, adherence to an age-based immunization schedule is appropriate.
Learning Objectives
Identify which patients receiving corticosteroids and other immunosuppressive agents are at higher risk for infection based on a patient’s individual characteristics and the immunosuppressive agent used
Choose the optimal and evidence-based infection prevention strategy to mitigate infection complications in patients receiving corticosteroids and other immunosuppressive agents
Clinical case
A 66-year-old woman presented with 2 weeks of easy bruising and epistaxis. She had chronic obstructive pulmonary disease (COPD), mild cognitive impartment, and essential hypertension. Her platelet count was 7000 per microliter (normal range, 150 000-400 000 per microliter); given her symptomatology, she was hospitalized for expedited workup and management. Physical and laboratory examinations were negative for rheumatologic or infectious causes of her thrombocytopenia. She was diagnosed with immune thrombocytopenic purpura (ITP). Her medications on admission were salmeterol, fluticasone, hydrochlorothiazide, lisinopril, and amlodipine. She lived alone. Her daughter lived 2 hours away but visits every weekend because her mother tends to confuse her medications. The patient is anxious about starting a new drug and the side effects that she might experience from it. She has had 3 hospitalizations for COPD exacerbation in the past 12 months; however, she had never been in the intensive care unit or been intubated. The medical team discussed a 4-day course of dexamethasone 40 mg once daily; however, the patient and her daughter argued against it given an episode of confusion the patient experienced while on dexamethasone during her last admission for COPD exacerbation. However, the patient stated that she has been on prednisone before and tolerated it well. The plan is now for IV immunoglobulin and prednisone taper over 4 to 8 weeks, starting at 1 mg/kg per day.
Introduction
The use of immunosuppressive therapies in the management of nonmalignant immune-mediated hematologic diseases, such as ITP, thrombotic thrombocytopenic purpura, autoimmune hemolytic anemia, antiphospholipid syndrome, and acquired coagulation factor deficiencies, lead to an increased risk for infections. Because these infectious complications can severely affect a patient’s outcome, preventive strategies, such as patient counseling, immunization, infectious disease screening, and antimicrobial prophylaxis, are essential tools in minimizing this risk. Here, we review the data on infectious risk and infectious disease prevention in patients with nonmalignant immune-mediated hematologic diseases treated with various immunosuppressive agents (corticosteroids, antimetabolites, calcineurin inhibitors, and cyclophosphamide [CP]) (see the Visual Abstract). We also highlight areas in which limited evidence exists and discuss our clinical approach given the existent knowledge base. The risk for infections associated with surgical and functional asplenia, as well as with the use of monoclonal antibodies (rituximab and eculizumab), is discussed elsewhere in this volume.63,64
What is this patient’s risk for infection?
The existing evidence regarding the risk of infection in patients with nonmalignant immune-mediated hematologic diseases treated with corticosteroids and other immunosuppressive agents is scarce. However, publications about patients with ITP or autoimmune rheumatologic disease undergoing immunosuppressive therapies report infection as a major cause of morbidity, treatment interruption, and/or discontinuation.1,2 When appraising the patient’s risk of infection, the interactions of various endogenous and exogenous risk factors have to be considered: (1) nonmalignant immune-mediated hematologic diseases are themselves chronic conditions driven by an already dysfunctional immune system; (2) a patient’s individual characteristics, such as advanced age (>65 years old), presence of preexisting comorbidities, polypharmacy (ie, ≥5 medications used daily), and risk of drug-drug interaction; the patient’s compliance with the immunosuppressive therapy and dose adjustments; compliance and accessibility to clinical and laboratory monitoring; and a patient’s rapid response to suspected infections; (3) specific immunosuppressive agents have particular mechanisms of causing immunosuppression, leading to an increased risk for infection with certain pathogens (eg, antimetabolites and risk for progressive multifocal leukoencephalopathy [PML]); and (4) higher risk for infection is seen in combination immunosuppressive therapy as opposed to single-agent therapy (Table 1).2-6 It is a challenge for the clinician to estimate the contribution of each of these risk factors to the overall infection risk because no study has addressed this clinical issue.
Table 1.
Factors associated with increased risk for infection
| Variable | Risk factors | |
|---|---|---|
| Patient-related factors | Age/functional status | Older age (>65 y old), poor functional status (frail) |
| Medical history | Preexisting comorbidities: chronic lung/liver disease, uncontrolled diabetes, severe malnutrition, IV drug use, hematologic cancer or any cancer on active chemotherapy or radiation treatment, chronic kidney disease on dialysis, asplenia, HIV/AIDS, primary immunodeficiencies, history of infections while on immunosuppressive therapies | |
| Socioeconomic status | Travel, high-cost burden, poor family/caregiver support, cognitive impairment. | |
| Therapy- and disease-related factors | Regimen chosen | Combination immunosuppressive therapy, high-cost burden |
| Length of treatment | Long-term/maintenance immunosuppressive therapy to maintain response | |
| Drug safety and side effect profile | Poor drug tolerability, polypharmacy (caveat: drug-drug interaction), strong need for laboratory/clinical monitoring while on treatment (eg, oral CP and risk for myelosuppression) | |
| Time to treatment | Shorter time from diagnosis to therapy (eg, patients with high disease burden) precluding appropriate immunization administration and/or infectious disease screening | |
| Dose response | Higher doses needed to achieve disease response | |
| Long-term efficacy | Frequent relapses, refractory disease |
With this in mind, the patient in our vignette can be considered as being high risk for infection based on the following risk factors: age > 65 years, chronic lung disease with multiple hospitalizations, cognitive impairment, polypharmacy, a nonnegligible risk for low adherence to medication, clinical/laboratory follow-up, and rapid response to suspected infection given her social/living situation; and a history of corticosteroid-induced side effects that led the team to choose a longer course of lower-dose oral corticosteroid.
Patient education and general recommendations
Preventing infectious complications in immunocompromised hosts requires engagement of the patient, their family, and caregivers. Patients and their close contacts must be empowered and enabled to perform self-care in a way that minimizes preventable harm. For example, the most cost-effective way to prevent transmission of infections is hand hygiene. Teaching patients to cleanse their hands and enabling family members to help them perform this simple task can decrease the load of pathogens responsible for infections.7 All members of the household should avoid interaction with the patient when they are experiencing an infectious process.7 Patients should always be advised to seek prompt medical attention during a febrile illness. Early management of animal bites is critical in immunocompromised patients.8 For travelers, the use of tick and mosquito repellents, netting while sleeping, antimalarial prophylaxis when traveling to endemic areas, and the advice from an infectious disease physician are all recommended.9
All patients should adhere to their age-based immunization schedule as per the Advisory Committee on Immunization Practices guidelines, including annual viral influenza vaccine and the herpes zoster vaccine for patients 50 years and older; the recombinant herpes zoster vaccine (SHINGRIX) is preferred over the live attenuated vaccine (Zostavax), as per Centers for Disease Control and Prevention (CDC) guidelines.10,11 The US Food and Drug Administration (FDA) advises that the administration of live or live attenuated vaccines is contraindicated in patients receiving immunosuppressive therapy (eg, corticosteroids ≥ 10 mg prednisone-equivalent dose [PEQ] daily or a cumulative dose > 700 mg PEQ in 3 months) and that vaccination should be deferred for ≥1 month after discontinuation of such therapy. Killed or inactivated vaccines and toxoids may be administered; however, the response to such vaccines cannot be predicted.10 HIV status should be known in any patient with a nonmalignant immune-mediated hematologic disease.
Clinical challenges and best practices
Patients with nonmalignant immune-mediated hematologic diseases may not adequately respond to first‐line therapy; often, no clear consensus exists as to when to stop first‐line therapy and what the optimal second‐line therapy should be after first-line treatment failure. This may lead to suboptimal management approaches, including prolonged exposure to treatments that may not be optimal for long‐term use, such as corticosteroids, which may fail to address symptoms and burden of disease and worsen health‐related quality of life.12
In this context, it is relevant that clinicians have a good understanding of available second‐line treatments to ensure the best use of therapeutic options and to avoid prolonged use of corticosteroids. Overall familiarity of the clinician with the therapy and rapidity of response appears to play a decisive role in long‐term management of patients with nonmalignant immune-mediated hematologic diseases.13,14 The use of evidence‐based practice guidance and guidelines and/or consulting a medical colleague expert in the field (eg, online tools such as “You Make the Call” by the American Society of Hematology) can be of help in challenging situations and can assist in minimizing complications and optimizing patient outcomes.
Corticosteroids
Corticosteroids exert a complex quantitative and qualitative immunosuppressive effect that induces cellular immunodeficiency and, consequently, increased patient susceptibility to infections. The three key corticosteroid effects leading to an altered immunologic response against pathogens are (1) impaired opsonization and phagocytic function increasing the risk for bacterial infections, (2) impaired T-cell migration and proliferation increasing the risk for mycobacterial, viral, and fungal infection, and (3) impaired eosinophilic proliferation with increased apoptosis, resulting in an increased risk for parasitic infection.15
Even though corticosteroids are commonly used in the management of various autoimmune diseases, little is known about an individual patient’s risk for infection associated with such treatment. A population-based cohort study using general practice records in the United Kingdom compared all adults who had been prescribed corticosteroids with adults who had not been prescribed them.16 Hazard ratios (HRs) were significantly higher among corticosteroid recipients vs nonrecipients for cutaneous cellulitis (2.21; 95% confidence interval [CI] .06-2.37), bloodstream infection (HR, 3.96; 95% CI, 3.19-4.93), local candidiasis (HR, 4.93; 95% CI, 4.60-5.29), and lower respiratory tract infections (HR, 5.42; 95% CI, 5.23-5.61) (P < .001 for all comparisons).16
Current conclusions concerning corticosteroid-related infection risk in nonmalignant immune-mediated hematologic diseases are largely derived from studies of patients with rheumatologic and inflammatory bowel diseases.17-20 Studies in patients aged 66 years and older with rheumatoid arthritis (after adjusting for disease severity, use of other immunosuppressive agents, and comorbidities) found an increased risk for serious bacterial infections with PEQ as low as 5 mg for 1 week (odds ratio, 1.03-3.96), as well as a dose-dependent (ie, >20 mg PEQ daily) and a duration-dependent (ie, > 5 mg PEQ chronically) stepwise increase in the risk of serious bacterial infections (odds ratio, 2.0-7.57).18,21,22 Likewise, in patients aged 66 years and older with inflammatory bowel disease, the use of corticosteroids alone resulted in an estimated fivefold relative risk for bacterial infections, a fourfold relative risk for other infections (eg, Strongyloides and tuberculosis), and 1.5-fold risk for viral infections.20
Factors associated with an increased risk for infection with systemic corticosteroids include age > 65 years; lower functional status; preexisting comorbidities, such as diabetes mellitus, lung disease (asthma, COPD), and malnutrition (low albumin); higher corticosteroids doses (≥20 mg PEQ daily), and longer duration of corticosteroid therapy (≥4-8 weeks).16–18 Although the absolute individual risk of infectious complications from corticosteroid use remains fairly small, the burden is significant at a population level because of the high frequency of corticosteroid use. Thus, most practitioners eventually encounter these complications during their career.
Although numerous opportunistic infections (eg, aspergillosis, nontuberculous mycobacterial disease, candidiasis, cryptococcosis) have been reported with the use of systemic corticosteroids, this section will focus on those for which data are most solid and for which implementation of infection-prevention strategies has demonstrated a lessening of their appearance and/or minimization of further complications. A summary of these infection complications and preventive strategies is presented in Table 2.17,23-28
Table 2.
Infectious complications and preventive strategies with the use of systemic corticosteroids
| Pathogen | Risk factors for infection | Preventive strategy |
|---|---|---|
| PJP | A. Corticosteroid dose ≥ 30 mg PEQ daily given for ≥4 wk B. Corticosteroids ≥ 15 mg to <30 mg PEQ daily given for ≥8 wk uninterrupted or in intermittent doses C. Combination of medium-dose corticosteroids (ie, ≥15 mg to <30 mg PEQ daily) and CP (oral or IV pulses) D. Corticosteroids ≥ 10 mg PEQ daily and ≥2 of the following: advanced age > 65 y, coexisting lung disease (eg, COPD, lung fibrosis), use of immunotherapeutics (eg, rituximab, anti-TNF). |
Antimicrobial prophylaxis: • For all patients in (A) through (D), PJP prophylaxis is indicated. • TMP/SMX, 1 single-strength tablet (80 mg of TMP and 400 mg of SMX) daily, or TMP/SMX, 1 double-strength tablet 3 times weekly. • If TMP/SMX intolerance or contraindicated, alternative therapies are atovaquone, dapsone, or once-monthly nebulized pentamidine. • For patients in (D), PJP prophylaxis should be continued until the corticosteroid dose is ≤5 mg PEQ daily. |
| HZ (shingles) | A. Advanced age > 60 y B. Corticosteroid dose > 7.5 mg to 10 mg PEQ C. History of recurrent shingles |
Immunization: • RZV (ie, SHINGRIX) preferred over ZVL (ie, Zostavax) • Indicated in all adults aged ≥ 50 y, including those who received ZVL in the past; had chickenpox or do not recall whether they had chickenpox; had shingles, but not an active flare at the time of vaccination; and have chronic comorbidities (eg, chronic renal failure, diabetes mellitus, autoimmune diseases, COPD) • In adults aged ≥ 50 y anticipating immunosuppression or currently on immunosuppressive therapy, important considerations are to vaccinate ideally ≥4 wk before treatment; okay in patients taking low-dose immunosuppressive therapy (eg, <20 mg/d prednisone or equivalent, or using inhaled or topical steroids, azathioprine, mycophenolate mofetil); and okay in patients who have recovered from an immunocompromising illness • Adults aged < 50 y: ACIP does not have a recommendation to administer either zoster vaccine to people younger than 50 y. However, based on the available evidence, clinicians may choose to administer a vaccine off-label, if, in their clinical judgment, they think that the vaccine is indicated (eg, history of shingles). The patient should be informed that the use is off-label and that efficacy and safety of the vaccine have not been tested in people younger than 50 y. Antimicrobial prophylaxis: • No evidence outside of the transplant setting exists on the use of antiviral prophylaxis. However, it might be reasonable that patients with history of recurrent shingles or heavily treated with immunosuppressive agent should consider antiviral prophylaxis. Doses as low as 400 mg of acyclovir daily have shown to an effective strategy in immunocompromised patients. |
| TB reactivation | A. Corticosteroid dose < 15 mg PEQ daily has a 2.8-fold increased risk B. Corticosteroid dose > 15 mg PEQ daily has a 7.7-fold increased risk |
TB screening testing: • Patients taking corticosteroids at a dose ≥ 10 mg PEQ daily for ≥4 wk should be screened for latent TB using tuberculin skin test or interferon-γ release assays; the latter is preferred in patients with altered T-cell function (eg, HIV/AIDS), history of BCG immunization, and ongoing corticosteroid therapy or other immunosuppressive agents • If positive test, refer to an infectious disease specialist |
| Disseminated SS hyperinfection syndrome | A. Major risk factor is provenance/travel history: tourists, military, and immigrant populations coming from high prevalence areas, such as Africa (Ghana, Zambia, Gabon, Sudan), Asia (Thailand, Cambodia), Central America (Guatemala), and South America (Peru, Venezuela, Brazil). B. There are no clear data on the dosage or duration of corticosteroid therapy that triggers the risk for severe strongyloidiasis. |
SS screening testing: • Given the available data, any patient coming from a high-risk area and scheduled to start corticosteroids at a dose > 10-15 mg PEQ daily for ≥4 wk should be screened with stool sample for ova and parasites and serum IgG against SS. Antimicrobial prophylaxis: • Given the poor sensitivity and high cost of SS screening, empiric therapy with ivermectin represents a safe and cost-effective approach in patients at high-risk for severe strongyloidiasis (ie, people walking barefoot in endemic areas). |
| HBV reactivation | A. High-dose corticosteroids (>20 mg PEQ daily) for >4 wk B. Chronic (≥8 wk) medium-dose corticosteroids (10-20 mg PEQ daily) |
HBV screening testing: • Patients in (A) and (B) need hepatitis B screening with anti-HBc and HBsAg. Results interpretation: • Patients in (A) or (B) with positive anti-HBc and positive HBsAg have a high risk for HBV reactivation (≥10% risk for reactivation). • Patients in (A) with positive anti-HBc, but negative HBsAg, have a moderate risk for HBV reactivation (1-10% risk of reactivation). Antimicrobial prophylaxis: • Patients with high risk for HBV reactivation require antiviral prophylaxis. • For patients with moderate risk for HBV reactivation, 2 options are available: preemptive therapy guided by serial HBV DNA monitoring, with antiviral therapy initiated as soon as HBV DNA becomes detectable, and routine prophylactic antiviral therapy. • Entecavir or tenofovir is the preferred agent because of the low risk of resistance. • Infectious disease input is encouraged. |
Data are from Youssef et al,17 Cavallasca et al,23 Dooling et al,24 Yun et al,25 Loomba and Liang,26 Katsuyama et al,27 and Center for Disease Control and Prevention.28
ACIP, Advisory Committee on Immunization Practices; anti-HBc, anti–hepatitis B core antibody; anti-TNF, anti–tumor necrosis factor inhibitors; BCG, bacillus Calmette-Guérin; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HZ, herpes zoster; IgG, immunoglobulin G; PJP, P jirovecii pneumonia; RZV, recombinant zoster vaccine; SMX, sulfamethoxazole; SS, Strongyloides stercoralis; TB, tuberculosis; TMP, trimethoprim; ZVL, zoster vaccine live.
Pneumocystis jirovecii pneumonia
Pneumocystis jirovecii pneumonia (PJP) is 1 of the most common causes of opportunistic lung infections in immunocompromised patients.29 The available evidence about the association between PJP and corticosteroids is basically derived from case series and single-center studies.17,30,31 Although no guidelines exist regarding what dose and duration of corticosteroids are necessary to trigger PJP in patients with nonmalignant immune-mediated hematologic diseases, the estimated incidence rate of PJP in patients with rheumatologic diseases has been shown to vary depending on the dose of corticosteroids used. For instance, patients receiving low-dose corticosteroids (ie, ≤15 mg PEQ daily) have an estimated 1-year incidence of 0.1 per 100 person-years, whereas those on moderate- and high-dose corticosteroids have estimated incidences of 0.5 and 0.75 per 100 person-years, respectively.30,31
Based on the available data, a clinician should consider PJP prophylaxis in patients at higher incidence for PJP, such as those on (1) a corticosteroid dose ≥ 30 mg PEQ daily given for ≥4 weeks, (2) a corticosteroid dose ≥ 15 mg to <30 mg PEQ daily given for ≥8 weeks, either uninterrupted or in intermittent doses, (3) a combination of medium-dose corticosteroids (ie, ≥15 mg to <30 mg PEQ daily) and CP (oral or IV pulses), and (4) corticosteroids ≥ 10 mg PEQ daily and ≥2 of the following: age > 65 years, coexisting lung disease (eg, COPD, lung fibrosis), or use of immunotherapeutics (eg, rituximab, anti–tumor necrosis factor).17,30,31 Because better therapies than long-term corticosteroids exist for the management of nonmalignant immune-mediated hematologic diseases, initiation of PJP prophylaxis at the time of diagnosis and initiation of corticosteroid treatment are typically not needed. However, a clinician should always assess a patient’s length of exposure and dose-dependent disease response to corticosteroids at each clinic visit and consider PJP prophylaxis if longer-term corticosteroid therapy is being pursued.
PJP prophylaxis with trimethoprim (TMP)/sulfamethoxazole (SMX) can be prescribed as 1 daily single-strength tablet (80 mg of TMP, 400 mg of SMX) or 1 double-strength tablet 3 times weekly. For patients who exhibit intolerance or contraindication (eg, glomerular filtration rate < 15 mL/min) to TMP/SMX, alternative therapies are atovaquone (1500 mg daily), dapsone (100 mg daily), or nebulized pentamidine (300 mg once monthly).17,31 No societal or other formal recommendation exists regarding the duration of prophylactic treatment. In our practice, we discontinue prophylaxis when the corticosteroid dose is <10 mg PEQ daily.
Herpes zoster
Herpes zoster (HZ) is caused by reactivation of latent varicella-zoster virus (VZV) in cranial nerve or dorsal root ganglia.25 Although usually presenting as a painful vesicular rash with a dermatomal distribution, immunocompromised individuals can develop disseminated disease, with vesicles spreading beyond the affected dermatome and the potential to affect other organs producing pneumonia, encephalitis, hepatitis, and retinitis.25 The incidence rate of HZ in healthy individuals has been reported to be 10.8 per 1000 person-years in people aged 60 to 69 years old and 6.7 per 1000 person-years in people aged 50 to 59 years.32,33 The risk for HZ associated with autoimmune conditions has been estimated at ∼1.5-fold to twofold higher than corresponding rates in healthy individuals, with a 2.37-fold increased risk for HZ in those receiving corticosteroids.16,25 Although the incidence of disseminated VZV infection in patients with nonmalignant immune-mediated hematologic diseases is unknown, it has been reported that 10% to 40% of immunocompromised individuals suffering from HZ could develop disseminated disease.34,35 Disseminated HZ infection has a 5% to 10% fatality rate.25 Fulminant visceral disseminated VZV infection without skin involvement has been described in a patient with autoimmune hemolytic anemia.36
As previously discussed, all patients aged ≥50 years should receive HZ immunization. However, current guidelines do not address indications in immunocompromised patients with regard to the novel recombinant vaccine and indications outside of the approved age of ≥50 years.10 Age > 60 years and systemic corticosteroid use > 7.5 to 10 mg PEQ daily have been associated with increased incidence and severity of HZ.17 However, young adults with autoimmune diseases who are on immunosuppressive therapy are an important group also at high risk.25 That said, and based on the available evidence, clinicians may choose to administer a vaccine off-label if, in their clinical judgment, the vaccine could be indicated (eg, young patient with history of shingles). The patient should be informed that the use is off-label and that efficacy and safety of the vaccine have not been tested in people younger than 50 years of age.
Finally, no evidence outside of the transplant setting exists on the use of antiviral prophylaxis; however, it might be reasonable to consider it in patients with history of shingles or patients heavily treated with immunosuppressive agents. Doses of oral acyclovir as low as 200 to 400 mg/d have shown effectiveness in preventing VZV reactivation in immunocompromised patients.37,38 Antiviral therapy should be initiated in all immunocompromised patients with active HZ. Immunocompromised hosts with disseminated zoster should be hospitalized for IV therapy.
Tuberculosis reactivation
Patients with latent tuberculosis (TB) infection on corticosteroids are at risk for conversion to active disease. The limited data available regarding the risk of TB reactivation with systemic corticosteroids comes from patients with rheumatologic diseases. Patients treated with <15 mg vs >15 mg PEQ daily have a 2.8-fold and 7.7-fold increased risk for TB reactivation, respectively.39 Those with TB reactivation are more likely to have received IV pulse-dose corticosteroids.40
The CDC recommends screening for latent TB infection in those who may need long-term immunosuppression (eg, ≥10 mg PEQ daily for >4 weeks).28 Latent TB infection screening should be performed with a tuberculin skin test or serum interferon-γ release assays; the latter is recommended in patients with altered T-cell function (eg, HIV/AIDS), history of bacillus Calmette-Guérin immunization, or ongoing immunosuppressive therapy.28 If a test is positive, the patient should be referred to an infectious disease specialist for appropriate management.
Strongyloides stercoralis infection
Strongyloides stercoralis (SS) is an intestinal nematode particular in its ability to produce chronic infection through cycles of autoinfection within the same host that can last for decades.41 Disseminated strongyloidiasis, or hyperinfection syndrome, is a lethal condition in which the parasite spreads from the intestinal tract to different organs causing septicemia and multiorgan failure. Immunosuppressive states, such as those produced by systemic corticosteroid use, are the major risk factor.41 A large systemic review on severe strongyloidiasis reported that 67% (163/244) of the cases occurred in patients on corticosteroid therapy, with a mortality rate of 62.7%; however, only 8% had autoimmune diseases (rheumatoid arthritis and lupus), and the study was not able to report the cumulative dosage and the duration of the corticosteroid treatment.42 In the United States, strongyloidiasis cases are seen in tourists, military, and immigrant populations coming from high-prevalence areas, such as Africa (Ghana, Zambia, Gabon, Sudan), Asia (Thailand, Cambodia), Central America (Guatemala), and South America (Peru, Venezuela, Brazil).36
Although no guidelines exist on the prevention of SS infection, given the available data, any patient coming from a high-risk area and scheduled to start a corticosteroid dose > 10 to 15 mg PEQ daily for ≥4 weeks should be screened with a stool sample for ova and parasites and serum immunoglobulin G against SS.42 In our practice, given the poor sensitivity and high cost of SS screening, empiric therapy with ivermectin represents a safe and cost-effective approach in patients at high risk for strongyloidiasis (ie, those who have lived in areas of high incidence and endorse a history of walking outside barefoot). Infectious disease input in such patients is warranted.
Hepatitis B virus reactivation
Hepatitis B virus (HBV) reactivation is defined as a sudden and rapid increase in HBV DNA level by ≥100-fold in patients with previously detectable HBV DNA or the reappearance of HBV DNA viremia in individuals who did not have viremia before the initiation of immunosuppressive or biological therapies.26 The timing of the onset and symptomatology of HBV reactivation is variable and depends on the host’s immunity, underlying disease, and the type of immunosuppressive therapy used.26 HBV reactivation may occur as early as 2 weeks from immunosuppressive therapy initiation or up to a year after the cessation of immunosuppression. Symptoms vary from mild constitutional symptoms, jaundice, abdominal pain and nausea/vomiting to fulminant liver failure.26 The risk of HBV reactivation can be divided broadly into high risk (rate of HBV reactivation ≥ 10%), moderate risk (1-10% rate), and low risk (<1% rate). This classification applies to those with positive anti–hepatitis B core antibody (anti-HBc) with a positive (or negative) hepatitis B surface antigen (HBsAg) and is based on the type of immunosuppressive therapy used. For instance, patients scheduled to receive chronic (≥8 weeks) medium-dose corticosteroids (10-20 mg PEQ daily) or high-dose corticosteroids (>20 mg PEQ daily) for ≥4 weeks are considered at high risk and should be screened for HBV.43 HBV viral screening should consist of anti-HBc and HBsAg in serum.
HBV prophylaxis is recommended to those with positive anti-HBc and positive HBsAg receiving chronic medium-dose corticosteroids and to those with positive anti-HBc and positive HBsAg receiving high-dose corticosteroids for ≥4 weeks (Table 2).26 On the other hand, patients with positive anti-HBc but negative HBsAg who are on high-dose corticosteroids are classified as having a moderate risk for reactivation and require careful monitoring.26 Antiviral drugs with a high barrier to resistance (ie, entecavir or tenofovir) are recommended.43 Treatment with antivirals should be continued for ≥6 months after discontinuation of corticosteroids.
Clinical case continued
The patient was started on prednisone, 60 mg by mouth daily, with a plan for a taper over 4 weeks. Prior to therapy, the patient underwent HIV testing, as well as screenings for latent TB infection with a serum interferon-γ release assay and HBV with serum anti-HBc and HBsAg. Tests results were negative. The patient stated that she had been vaccinated against shingles 2 years ago and was told it was with the new shingles vaccine. She was started on TMP/SMX double-strength tablets 3 times weekly for PJP prophylaxis. After 2 weeks on therapy, she had a robust response, with a platelet count of 225 000 per microliter. However, after 2 weeks of being on a corticosteroid taper, she returned to the clinic with 2 days of easy bruising while on 10 mg of prednisone. Her platelet count during that visit was 15 000 per microliter. Over the next year, she received rituximab with no response, as well as trials of eltrombopag and romiplostim that produced intermittent spikes in her platelet count but not a sustained response. Splenectomy could not be performed because of her poor lung function. She remained dependent on prednisone, 20 mg daily, to maintain a platelet count of 20 000 to 30 000 per microliter. Following the American Society of Hematology 2019 evidence-based ITP management recommendations that discourage the prolonged use of corticosteroids, a new regimen with mycophenolate mofetil (MMF), 500 mg by mouth twice daily, is planned.
Other immunosuppressive therapies
A range of immunosuppressive drugs (eg, azathioprine [AZA], MMF, cyclosporine, and CP), drug combinations, and dosing regimens are used to treat relapsed/refractory nonmalignant immune-mediated hematologic diseases, all of which are off-label for these disorders.13,44-46 A summary of recommendations is presented in Table 3.47-51
Table 3.
Infectious complications and preventive strategies with the use of AZA, MMF, cyclosporine, and CP
| Drug | Associated infection | Preventive strategy |
|---|---|---|
| AZA/MMF | Recognized association: • Virus: JC virus, cytomegalovirus, VZV Reported cases: • Bacteria: Listeria, Mycobacterium spp. • Viral: BK virus • Fungi: Cryptococcus, Aspergillus, PJP • Parasite: Toxoplasma |
Clinical evaluation: • In patients managed with antimetabolites and presenting with new-onset neurological symptoms such as hemiparesis, apathy, confusion, cognitive deficiencies, ataxia, blurry vision or loss of vision, severe otalgia or hearing loss, need evaluation for a neurotropic infection (eg, PML, HZ reactivation, toxoplasmosis, Cryptococcus). • Brain imaging and neurology consultation are recommended in those with neurologic symptoms. Immunization: • HZ immunization is recommended and as stated in Table 2. |
| Cyclosporine | Recognized association: • Virus: cytomegalovirus in transplanted patients Reported cases: • Bacteria: Gram-negative sepsis • Virus: Herpes simplex, VZV |
• No evidence outside of the transplant setting exists on the use of preventive strategies to minimize opportunistic infections. |
| CP | Recognized association: • Infections associated with neutropenia (common bacterial infection) Reported cases: • Bacterial: TB • Fungal: PJP, Aspergillus • Parasitic: SS |
Laboratory testing: • Routine blood cell counts. Therapy should not be administered to patients with an absolute neutrophil count ≤ 1500/μL and/or platelets < 50 000/μL. Antimicrobial prophylaxis: • Antimicrobial prophylaxis against bacterial, fungal, or viral infection might be considered in certain cases of neutropenia and at the discretion of the managing physician. • In case of neutropenic fever, antibiotic therapy is indicated, as well as consideration for growth factors, especially in patients considered to be at increased risk for neutropenia complications (eg, elderly patients). • PJP prophylaxis in patients treated with combination CP and moderate-dose corticosteroids (ie, ≥15 mg to <30 mg PEQ daily). PJP prophylaxis can be discontinued once PEQ ≤ 5 mg daily. |
Antimetabolites: AZA and MMF
Infectious complications reported with the use of antimetabolites are bacterial (common bacteria and atypical bacterial infections, including Listeria monocytogenes, Mycobacterium spp.)52,53, fungal (Cryptococcus, Aspergillus, Mucor, PJP)47-49, parasitic (Toxoplasma)54, and viral reactivation (disseminated HZ, JC virus, polyomavirus-associated nephropathy-BK virus infection).48,49 Although AZA and MMF carry a “black-box” warning for the development of progressive multifocal leukoencephalopathy (PML), an opportunistic demyelinating disease caused by the JC virus, the exact incidence rate attributed to these drugs is unknown.48,49 However, the incidence rate of PML in patients with autoimmune diseases other than rheumatoid arthritis and lupus erythematous systemic who are on immunosuppressive therapy is estimated to be 2 per 100 000 people.55 Hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia are the most frequent clinical features observed in PML. Additionally, disseminated HZ infection has been reported in patients treated with MMF outside of the transplant setting.56,57
No specific recommendations are available for the prevention of opportunistic infections with the use of antimetabolites. The FDA recommends that a diagnosis of PML be considered in any patient treated with AZA or MMF presenting with new-onset neurological manifestations and to consider consultation with a neurologist as clinically indicated.56,57 All immunosuppressive drugs should be discontinued during an episode of infection.
Cyclosporine
Cyclosporine selectively impairs T-cell function, increasing a patient’s risk for localized and/or generalized infections (viral, bacterial, fungal, or parasitic).50 Evidence on the risk of infection in nonmalignant immune-mediated hematologic patients is lacking. A study of cyclosporine in psoriatic patients reported a low risk for viral reactivation compared with transplanted patients.58 However, no head-to-head comparison on the safety of cyclosporine vs other immunosuppressant (eg, antimetabolites) has been reported. Correspondingly, no recommendations exist on the prevention of opportunistic infections in patients with nonmalignant immune-mediated hematologic disease treated with cyclosporine.
Cyclophosphamide
CP is an alkylating agent that is capable of inducing DNA single-strand breaks, thus preventing cells from dividing. CP is typically administered IV in pulses or orally as a continuous treatment.13 A common side effect is myelosuppression with leukopenia and neutropenia, which may lead to serious and sometimes fatal infections, including bacterial, fungal, viral, protozoal, and parasitic infections. Bacterial pneumonia is a common infection (up to 30% of infections); however, fatal cases are uncommon. Serious infections have been reported with CP in patients receiving concomitant corticosteroids.23,51,59 No difference in the risk for infection has been found in patients receiving IV vs oral CP.60
The FDA recommends routine blood cell counts in patients treated with CP. CP should not be administered to patients with absolute neutrophil count ≤ 1500 per microliter and/or platelet count < 50 000 per microliter. Antimicrobial prophylaxis might be considered in certain cases of neutropenia and at the discretion of the managing physician. In case of neutropenic fever, antibiotic therapy is indicated, as well as consideration for growth factors, especially in patients considered to be at increased risk for neutropenia complications (eg, patients aged ≥66 years). Additionally, we recommend PJP prophylaxis in patients treated with CP and moderate-dose corticosteroids (ie, ≥15 mg to <30 mg PEQ daily). PJP prophylaxis can be discontinued once PEQ is ≤5 mg daily.59
Clinical case continued
The patient’s platelet count improved after 3 months on MMF monotherapy. At this time, plan of care include monitoring for any new-onset neurological manifestations (hemiparesis, apathy, confusion, cognitive deficiencies, ataxia, blurry vision or loss of vision, severe otalgia, or hearing loss), as well as any signs of viral reactivation, such as skin manifestations from shingles.
COVID-19 and immunosuppressive therapy
The effect of the COVID-19 pandemic on medical care for conditions such as nonmalignant hematologic diseases is difficult to quantify. There is limited evidence regarding the use of immunosuppressive therapy (eg, corticosteroids) and the theoretical risk of increasing susceptibility to COVID-19 infection. Similarly, the role of corticosteroids in mitigating COVID-19 hyperinflammatory syndrome remains controversial.
As of 30 June 2020, there are no data on the risk of COVID-19 infection and its consequences on clinical outcomes in patients with nonmalignant immune-mediated hematologic diseases who are undergoing immunosuppressive therapy with corticosteroids, antimetabolites, cyclosporine, and CP. The COVID-19 Global Rheumatology Alliance Provider Registry houses data on >1400 COVID-19 patients with inflammatory rheumatologic diseases; preliminary data were released for 600 SARS-Cov-2+ patients.61 The use of ≥10 mg PEQ was associated with a more severe COVID-19 disease course and increased risk for hospitalization (odds ratio [OR], 2.05; 95% CI, 1.06-3.96; P = .03) compared with lower corticosteroid doses (OR, 1.03; 95% CI, 0.64-1.66; P = .91). Conversely, the use of disease-modifying antirheumatic drugs was associated with a lower hospitalization rate (OR, 0.46; 95% CI, 0.22-0.93; P = .03). Age > 65 years (OR, 2.56; 95% CI, 1.62-4.04; P < .01) and common comorbidities, such as hypertension and cardiovascular disease (OR, 1.86; 95% CI, 1.23-2.81; P < .01), lung disease (OR, 2.48; 95% CI, 1.55-3.98; P < .01), diabetes (OR, 2.61; 95% CI, 1.39-4.88; P < .01), and renal disease (OR, 3.02; 95% CI, 1.21-7.54; P = .02), were also linked to an increased risk for hospitalization.61
On 8 June 2020, the RECOVERY (Randomized Evaluation of COVid-19 thERapY) trial, an established randomized clinical trial to test a range of potential treatments for COVID-19, reported preliminary data on 2104 patients randomized to receive dexamethasone, 6 mg once per day (by mouth or by IV injection) for 10 days, vs 4321 patients randomized to usual care alone.62 Dexamethasone was associated with a lower death rate in ventilated patients (29% vs 40.7%; rate ratio [RR], 0.65; 95% CI, 0.48-0.88; P = .0003), as well as a lower death rate in patients receiving oxygen only without mechanical ventilation (21.5% vs 25%; RR, 0.80; 95% CI, 0.67-0.96; P = .0021). There was no benefit among patients who did not require respiratory support (17% vs 13.2%; RR, 1.22; 95% CI, 0.86-1.75; P = .14).
Acknowledging the limitations of existing data and the fact that empirical decision making might be necessary under the current pandemic, the following are some recommendations to consider when evaluating patients undergoing immunosuppressive therapy for a nonmalignant hematologic disease: (1) patients on low-dose corticosteroids (ie, <10 mg PEQ daily) might not need modification of their current regimen if their hematologic disease is controlled; (2) in patients on higher doses of corticosteroids (ie, >10 mg PEQ daily), consideration of a more effective second-line therapy might allow for tapering and, possibly, discontinuation of the corticosteroid; and (3) in newly diagnosed patients with a nonmalignant hematologic disease or for those experiencing disease relapse but known to be responsive to corticosteroids, a short course of high-dose corticosteroid therapy (1-5 days) might be reasonable to treat the acute episode. In all cases, an approach based on individual patient factors (eg, urgency of need for immunosuppressive therapy, patient comorbidities, measures to minimize exposure to SARS-Cov-2 infection) is highly encouraged. Lastly, prospective studies are needed to better understand the impact of COVID-19 on the management of patients with nonmalignant hematologic diseases.
Conclusions
The use of immunosuppressive therapy in the management of nonmalignant immune-mediated hematologic diseases carries a risk for infection. Although the absolute risk for the individual patient remains small, the burden of such complications at a population level can be significant because of the frequent use of immunosuppressive agents in clinical practice. Patient education, immunization, laboratory screening, and antimicrobial prophylaxis can diminish the risk. Adherence to these preventive strategies is a key element to prevent infectious complications.
References
- 1.Ovalles-Bonilla JG, Fernández-Berrizbeitia O, Martínez-Barrio J, et al. Causes of death in 350 patients with systemic autoimmune rheumatic diseases. Paper presented at 2017 American College of Rheumatology/Association of Rheumatology Professionals Annual Meeting. 7 November 2017. San Diego, CA.
- 2.Portielje JEA, Westendorp RGJ, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97(9):2549-2554. [DOI] [PubMed] [Google Scholar]
- 3.Ekstrand C, Linder M, Cherif H, Kieler H, Bahmanyar S. Increased susceptibility to infections before the diagnosis of immune thrombocytopenia. J Thromb Haemost. 2016;14(4):807-814. [DOI] [PubMed] [Google Scholar]
- 4.Bouwman L, Eeltink CM, Visser O, Janssen JJWM, Maaskant JM. Prevalence and associated factors of medication non-adherence in hematological-oncological patients in their home situation. BMC Cancer. 2017;17(1):739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford). 2013;52(1):53-61. [DOI] [PubMed] [Google Scholar]
- 6.Fox SW, Angus B, Minassian A, Rawlinson TA. Infection in the immunocompromised host. In: Warrell DA, Cox TM, Firth JD eds. Oxford Textbook of Medicine. 5th ed. Oxford, UK: Oxford University Press; 2018:25-34. [Google Scholar]
- 7.Rubin LG, Schaffner W. Clinical practice. Care of the Asplenic Patient. N Engl J Med. 2014;371(4):349-356. [DOI] [PubMed] [Google Scholar]
- 8.Steele RW. Should immunocompromised patients have pets? Ochsner J. 2008;8(3):134-139. [PMC free article] [PubMed] [Google Scholar]
- 9.Patel RR, Liang SY, Koolwal P, Kuhlmann FM. Travel advice for the immunocompromised traveler: prophylaxis, vaccination, and other preventive measures. Ther Clin Risk Manag. 2015;11:217-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. General Best Practice Guidelines for Immunization. Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed 7 July 2020.
- 11.Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087-2096. [DOI] [PubMed] [Google Scholar]
- 12.Harris E, Tiganescu A, Tubeuf S, Mackie SL. The prediction and monitoring of toxicity associated with long-term systemic glucocorticoid therapy. Curr Rheumatol Rep. 2015;17(6):513. [DOI] [PubMed] [Google Scholar]
- 13.Cuker A, Neunert CE. How I treat refractory immune thrombocytopenia. Blood. 2016;128(12):1547-1554. [DOI] [PubMed] [Google Scholar]
- 14.Cuker A. Transitioning patients with immune thrombocytopenia to second-line therapy: challenges and best practices. Am J Hematol. 2018;93(6):816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cutolo M, Seriolo B, Pizzorni C, et al. . Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153-155. [DOI] [PubMed] [Google Scholar]
- 16.Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med. 2016;13(5):e1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon WG, Suissa S, Hudson M. The association between systemic glucocorticoid therapy and the risk of infection in patients with rheumatoid arthritis: systematic review and meta-analyses. Arthritis Res Ther. 2011;13(4):R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulis G, Palmaro A, Sailler L, Lapeyre-Mestre M. Corticosteroid risk function of severe infection in primary immune thrombocytopenia adults. A nationwide nested case-control study. PLoS One. 2015;10(11):e0142217 doi:10.1371/journal.pone.0142217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brassard P, Bitton A, Suissa A, Sinyavskaya L, Patenaude V, Suissa S. Oral corticosteroids and the risk of serious infections in patients with elderly-onset inflammatory bowel diseases. Am J Gastroenterol. 2014;109(11):1795-1802, quiz 1803. [DOI] [PubMed] [Google Scholar]
- 21.Widdifield J, Bernatsky S, Paterson JM, et al. . Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(3):353-361. [DOI] [PubMed] [Google Scholar]
- 22.Dixon WG, Abrahamowicz M, Beauchamp ME, et al. . Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis. 2012;71(7):1128-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavallasca JA, Costa CA, Maliandi MR, Contini LE, Fernandez de Carrera E, Musuruana JL. Severe infections in patients with autoimmune diseases treated with cyclophosphamide. Reumatol Clin. 2015;11(4):221-223. doi:10.1016/j.reumae.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 24.Dooling KL, Guo A, Patel M, et al. . Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun H, Yang S, Chen L, et al. . Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68(9):2328-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsuyama T, Saito K, Kubo S, Nawata M, Tanaka Y. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis Res Ther. 2014;16(1):R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Latent Tuberculosis Infection. A Guide for Primary Health Care Providers. Available at: https://www.cdc.gov/tb/publications/ltbi/default.htm. Accessed 7 July 2020.
- 29.Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34(8):1098-1107. [DOI] [PubMed] [Google Scholar]
- 30.Chew L-C, Maceda-Galang LM, Tan YK, Chakraborty B, Thumboo J. Pneumocystis jirovecii pneumonia in patients with autoimmune disease on high-dose glucocorticoid. J Clin Rheumatol. 2015;21(2):72-75. [DOI] [PubMed] [Google Scholar]
- 31.Park JW, Curtis JR, Kim MJ, Lee H, Song YW, Lee EB. Pneumocystis pneumonia in patients with rheumatic diseases receiving prolonged, non-high-dose steroids-clinical implication of primary prophylaxis using trimethoprim-sulfamethoxazole. Arthritis Res Ther. 2019;21(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmader KE, Levin MJ, Gnann JW Jr, et al. . Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012;54(7):922-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oxman MN, Levin MJ, Johnson GR, et al. ; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. [DOI] [PubMed] [Google Scholar]
- 34.Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9(3):361-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koc Y, Miller KB, Schenkein DP, et al. . Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000;6(1):44-49. [DOI] [PubMed] [Google Scholar]
- 36.Akiyama M, Yoshifuji K, Fukuda T, et al. . Fulminant visceral disseminated varicella-zoster virus infection without skin involvement in a patient with autoimmune hemolytic anemia on prednisolone therapy [in Japanese]. Rinsho Ketsueki. 2016;57(4):467-471. [DOI] [PubMed] [Google Scholar]
- 37.Kawamura K, Wada H, Yamasaki R, et al. . Prophylactic role of long-term ultra-low-dose acyclovir for varicella zoster virus disease after allogeneic hematopoietic stem cell transplantation. Int J Infect Dis. 2014;19(1):26-32. [DOI] [PubMed] [Google Scholar]
- 38.Fei N, Shah N, Cumpston A, et al. . Low-dose acyclovir prophylaxis for varicella zoster reactivation in autologous hematopoietic cell transplantation recipients. Clin Hematol Int. 2019;1(2):101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55(1):19-26. [DOI] [PubMed] [Google Scholar]
- 40.Tam L-S, Li EK, Wong S-M, Szeto C-C. Risk factors and clinical features for tuberculosis among patients with systemic lupus erythematosus in Hong Kong. Scand J Rheumatol. 2002;31(5):296-300. [DOI] [PubMed] [Google Scholar]
- 41.Schär F, Trostdorf U, Giardina F, et al. . Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buonfrate D, Requena-Mendez A, Angheben A, et al. . Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;13(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy [published correction appears in Gastroenterology. 2015;148(2):455]. Gastroenterology. 2015;148(1):215-219, quiz e16-e17. [DOI] [PubMed] [Google Scholar]
- 44.Crowther M, Chan YLT, Garbett IK, Lim W, Vickers MA, Crowther MA. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118(15):4036-4040. [DOI] [PubMed] [Google Scholar]
- 45.Arnold DM, Nazi I, Santos A, et al. . Combination immunosuppressant therapy for patients with chronic refractory immune thrombocytopenic purpura. Blood. 2010;115(1):29-31. [DOI] [PubMed] [Google Scholar]
- 46.Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood. 2017;129(22):2971-2979. [DOI] [PubMed] [Google Scholar]
- 47.Gibson RH, Evans RJ, Hotham R, et al. . Mycophenolate mofetil increases susceptibility to opportunistic fungal infection independent of lymphocytes. bioRxiv. 2018; [Google Scholar]
- 48.Prometheus Laboratories Inc. IMURAN® (azathioprine) (Product information). https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/016324s037,017391s016lbl.pdf. Accessed 7 July 2020.
- 49.Roche Laboratories Inc. CellCept® (mycophenolate mofetil capsules) (Product information). https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050722s021,050723s019,050758s019,050759s024lbl.pdf. Accessed 7 July 2020.
- 50.Kim JH, Perfect JR. Infection and cyclosporine. Rev Infect Dis. 1989;11(5):677-690. [DOI] [PubMed] [Google Scholar]
- 51.Baxter. Cyclophosphamide (Highlights of prescribing information). https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/012141s090,012142s112lbl.pdf. Accessed 7 July 2020.
- 52.Del Pozo JL, de la Garza RG, de Rada PD, Ornilla E, Yuste JR. Listeria monocytogenes septic arthritis in a patient treated with mycophenolate mofetil for polyarteritis nodosa: a case report and review of the literature. Int J Infect Dis. 2013;17(2):e132-e133. [DOI] [PubMed] [Google Scholar]
- 53.Teh CL, Kong KO, Chong AP, Badsha H. Mycobacterium haemophilum infection in an SLE patient on mycophenolate mofetil. Lupus. 2002;11(4):249-252. [DOI] [PubMed] [Google Scholar]
- 54.Bernardo DR Jr, Chahin N. Toxoplasmic encephalitis during mycophenolate mofetil immunotherapy of neuromuscular disease. Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kartau M, Sipilä JO, Auvinen E, Palomäki M, Verkkoniemi-Ahola A. Progressive multifocal leukoencephalopathy: current insights. Degener Neurol Neuromuscul Dis. 2019;9:109-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hegde S, Annamalai R, Biswas J. Extensive herpes zoster involvement following mycophenolate mofetil therapy for sarcoidosis. J Ophthalmic Inflamm Infect. 2012;2(1):47-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saha M, Black MM, Groves RW. Risk of herpes zoster infection in patients with pemphigus on mycophenolate mofetil. Br J Dermatol. 2008;159(5):1212-1213. [DOI] [PubMed] [Google Scholar]
- 58.Colombo D, Chimenti S, Grossi P, et al. . Prevalence of past and reactivated viral infections and efficacy of cyclosporine A as monotherapy or in combination in patients with psoriatic arthritis--synergy study: a longitudinal observational study. BioMed Res Int. 2014;2014:941767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cortazar FB, Muhsin SA, Pendergraft III WF, et al. . Combination therapy with rituximab and cyclophosphamide for remission induction in ANCA vasculitis. Kidney Int Rep. 2017;3(2):394-402. doi:10.1016/j.ekir.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Opastirakul S, Chartapisak W. Infection in children with lupus nephritis receiving pulse and oral cyclophosphamide therapy. Pediatr Nephrol. 2005;20(12):1750-1755. [DOI] [PubMed] [Google Scholar]
- 61.Yazdany J. COVID-19 epidemiology, transmission and insights from global registry data. In: San Francisco: American College of Rheumatology State-of-the-Art Clinical Symposium. May 16-17, 2020 (virtual meeting); 2020. https://www.rheumatology.org/Announcements/fbclid/IwAR3EUYQwlItkJ6gVrMbLAaC9TIG8BZR1QxpBpZc8ACBOmDc612hGlZ4T0LA.
- 62.University of Oxford. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. Available at: http://www.ox.ac.uk/news/2020-06-16-low-cost-dexamethasone-reduces-death-one-third-hospitalised-patients-severe. Accessed 7 July 2020.
- 63.Lee GM. Preventing infections in children and adults with asplenia. Hematology Am Soc Hematol Educ Program. 2020;2020:328-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engel ER, Walter JE. Rituximab and eculizumab when treating nonmalignant hematologic disorders: infection risk, immunization recommendations, and antimicrobial prophylaxis needs. Hematology Am Soc Hematol Educ Program. 2020;2020:312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]


