Figure 3.
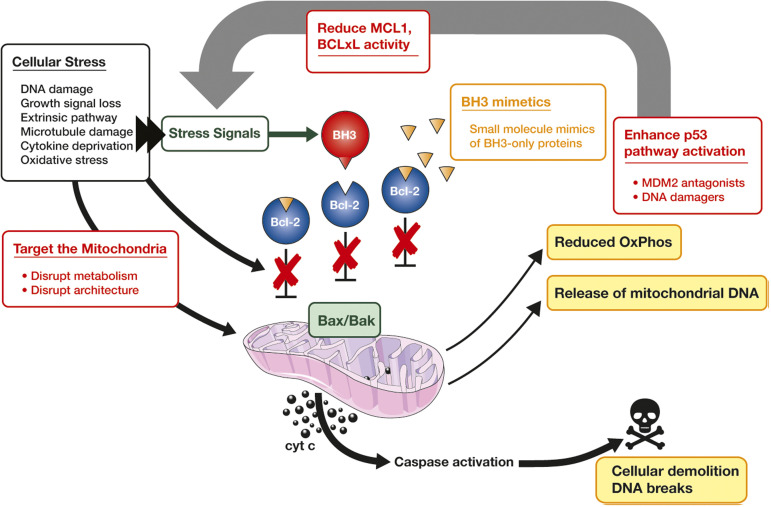
The anticancer effect of venetoclax theoretically can be enhanced through rational combination with other targeted therapies. This cartoon builds on the illustration of the mechanism of action of venetoclax in Figure 1 to highlight opportunities for enhancing apoptosis. A major avenue for amplifying the proapoptotic signal is to reduce the expression or activity of other prosurvival BCL2-like proteins (eg, MCL1 or BCLxL; red box, top center). This can be achieved directly by adding selective inhibitors of these proteins. Examples where this has been demonstrated preclinically32,54,63 and are being explored in clinical trials include AML, ALL, and mantle cell lymphoma. Reduction in prosurvival protein function can also be achieved indirectly via induction of their natural antagonists (eg, NOXA which antagonizes MLC1 and BCL2A1) by enhancing activity of the TP53 pathway through DNA damage or inhibition of MDM264 (red box, right side). These strategies are being explored clinically in lymphomas and AML. Preclinical evidence further indicates that killing can be augmented through direct targeting of mitochondrial structures and functions, such as energy production (red box, left side). This has been demonstrated particularly, but not only, in AML.15,16 A partial explanation of the enhanced efficacy of the azacitidine-venetoclax combination in AML includes disruption to energy metabolism.65 The cartoon also depicts how combinatorial approaches can both amplify the proapoptotic effect upstream of BAX/BAK and also reduce the threshold for mitochondrial vulnerability to BAX/BAK activation. To maximize the therapeutic index for any of these combination approaches, each will need to be tailored to the specific vulnerabilities of individual diseases, and biomarkers may prove advantageous in this regard.