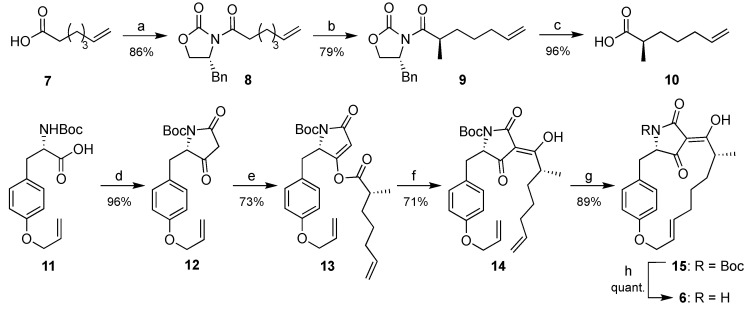
Scheme 1.
Synthesis of macrocidin Z (6). Reagents and conditions: (a) DCC, DMAP, (R)-benzyl-2-oxazolidinone, CH2Cl2, 23 h; (b) 1. NaHMDS, THF, −78 °C, 30 min, 2. MeI, 4.5 h; (c) LiOH, H2O2, THF/H2O (2:1); (d) Meldrum´s acid, DMAP, EDC∙HCl, CH2Cl2, rt, 2 h; (e) 10, DMAP, EDC∙HCl, CH2Cl2, 0 °C, rt, 2 h; (f) NEt3, DMAP, CH2Cl2, rt, 24 h; (g) Grubbs II catalyst, CH2Cl2, ∆, 15 h; (h) TFA, CH2Cl2, rt, 15 min. DCC = dicyclohexylcarbodiimide; DMAP = dimethylaminopyridine; NaHMDS = sodium hexamethyldisilazanide; THF = tetrahydrofuran; EDC = 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; TFA = trifluoroacetic acid.