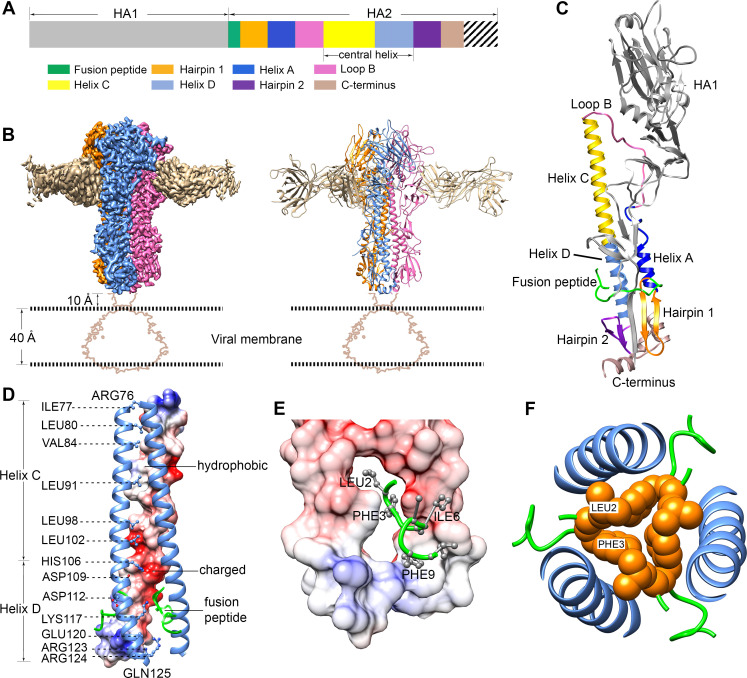
Fig 1. Cryo-EM structure of the influenza virus HA in complex with Fab F005-126 (HA-Fab) at pH 7.8.
(A) A schematic diagram showing the domain organization of the influenza virus HA. HA1 subunit (9–324), gray. Fusion peptide (1–20), green. Hairpin 1 (21–38), orange. Helix A (39–56), blue. Loop B (57–75), hot pink. Helix C (76–105), yellow. Helix D (106–125), cornflower blue. Hairpin 2 (126–141), purple. C-terminus (142–172), brown. The transmembrane domain and the cytoplasmic tail (173–222) are represented as stripe lines. (B) Surface-shadowed (left) and ribbon diagrams (right) showing the 2.8 Å cryo-EM structure of the HA-Fab at pH 7.8. The map is contoured at 8 σ. The disordered transmembrane domain and the bound detergent micelle are visible only at a lower map contour level, and the profile of the corresponding part at a contour level of 3 σ is therefore indicated in the diagram by brown lines. The three HA1/HA2 heterodimers are colored hot pink, cornflower blue and orange, respectively. The bound Fabs are colored tan. (C) Ribbon diagrams showing the structure elements of a HA1/HA2 heterodimer. The structure elements are labeled and colored the same as in (A). (D) Ribbon and surface diagrams showing the central helices. Side chains of the residues in the core of one central helix are shown in balls and sticks. The surface of one central helix is shown and colored according to the surface electrostatic potentials, with blue representing positive electrostatic potential and red representing negative electrostatic potential. The ribbons of the fusion peptides are colored green. (E) Ribbon and surface diagrams showing one fusion peptide in the surface pocket between the Helix Ds. The surface is colored according to the surface electrostatic potentials. The backbone of the fusion peptide is colored green, and the sidechains of the hydrophobic residues are colored gray. (F) Ribbon diagrams showing the hydrophobic core constituted by the hydrophobic fusion peptide terminal residues. The backbone of the fusion peptide is shown in green. The N-terminal hydrophobic residues of the fusion peptide are shown as orange spheres. The central helices are colored cornflower blue.