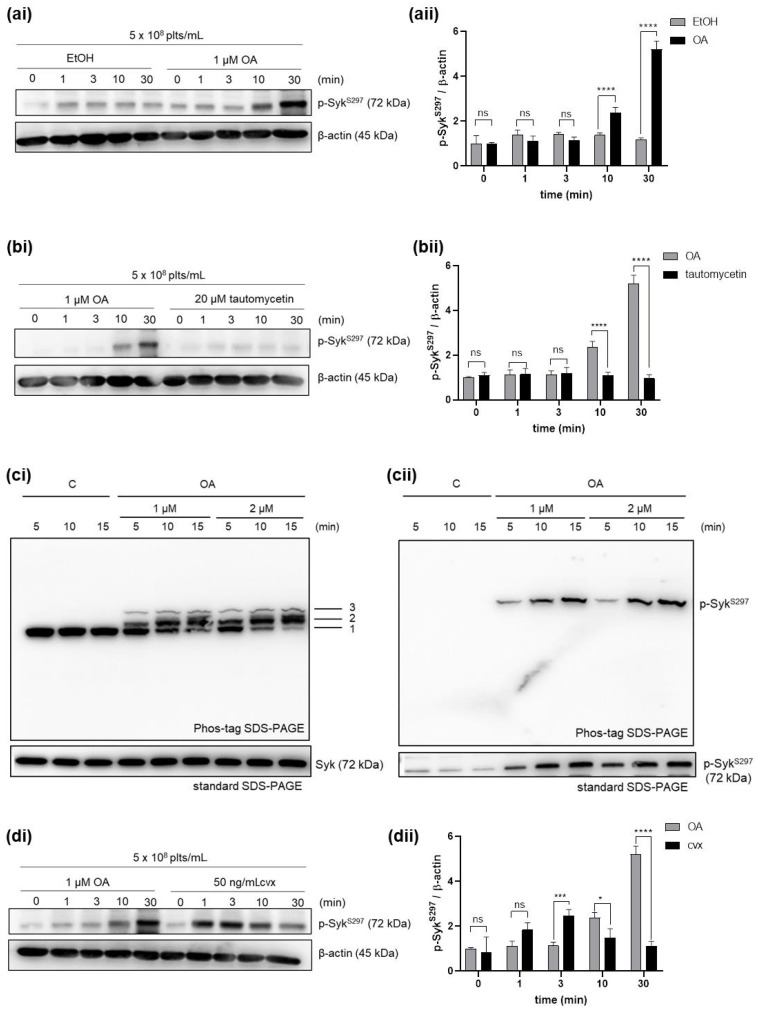
Figure 1.
Specific protein phosphatase 2A (PP2A) inhibition elevates Syk S297 phosphorylation in human platelets. Washed human platelets were incubated, as indicated, (a,c) with the vehicle ethanol (EtOH; C: vehicle control), (a–d) with 1 µM/2 µM of the PP2A inhibitor okadaic acid (OA), or with (b) 20 µM of the PP1 inhibitor tautomycetin, or were stimulated with (d) 50 ng/mL convulxin (Cvx) at 37 °C. Samples were analyzed by (a–d) conventional SDS-PAGE (8.0% w/v polyacrylamide), or by (ci,cii) phos-tag SDS-PAGE (6.0% w/v polyacrylamide and 35 µM Zn2+-phos-tag). Time-dependent phosphorylation of Syk S297 was monitored and analyzed by immunoblotting compared to β-actin. (ai, bi, di) Representative western blots of Syk S297 in the presence of (aii) OA, (bii) tautomycetin, or (dii) Cvx. Quantitative data of Syk S297 in the presence of (aii) OA, (bii) tautomycetin, or (dii) Cvx are represented as means ± S.D from three technical experiments, with platelets from the same donor. * p < 0.1, *** p < 0.001, **** p < 0.0001, ns p > 0.05.