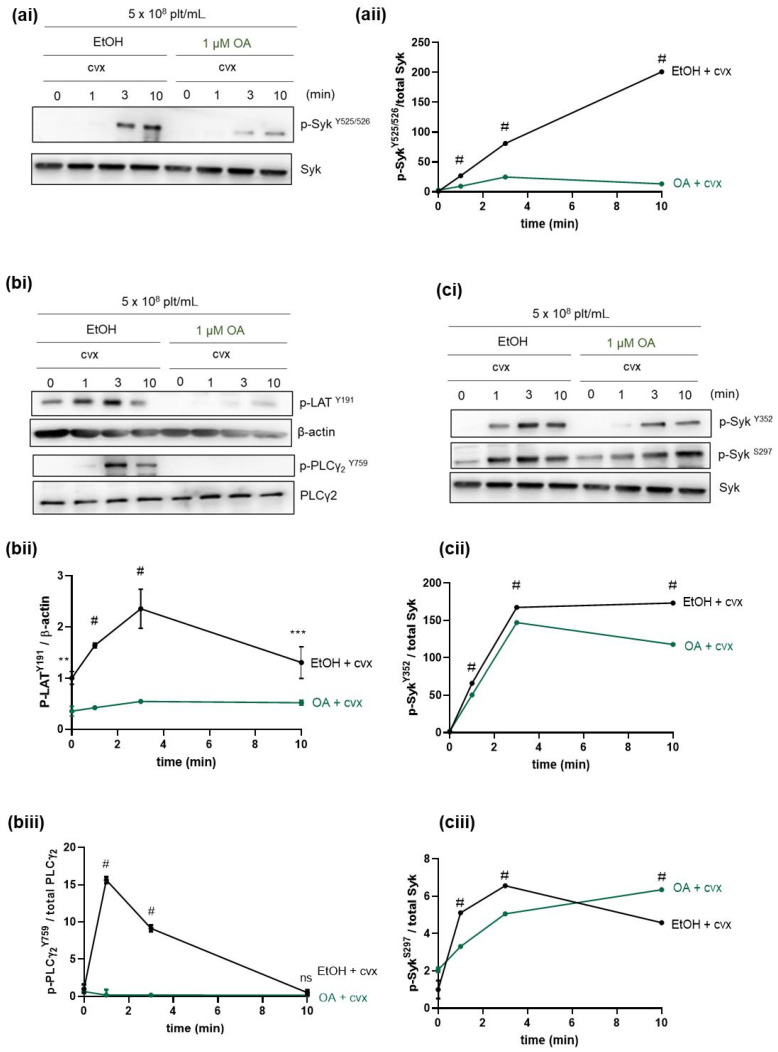
Figure 3.
PP2A inhibition significantly reduced Cvx-stimulated Syk Y525/526 phosphorylation (kinase domain) and phosphorylation of Syk substrates LAT and PLCγ2, with minimal effects on the phosphosites Syk Y352 and S297 (both interdomain-B). Washed human platelets were pre-incubated for 10 min with vehicle control (EtOH) or with 1 µM of the PP2A inhibitor OA prior to stimulation with 50 ng/mL Cvx. Samples for western blot analysis were taken at the indicated time points after the addition of Cvx and mixed with Laemmli buffer. Time-dependent phosphorylation of (a) Syk Y525/526 (b), LAT Y191 and PLCγ2, and (c) Syk Y352 and S297 was analyzed by immunoblotting compared to the corresponding loading control. (ai, bi, ci) Representative western blots of Syk Y525/526 (ai), LAT Y191, PLCγ2 Y759 (bi), Syk Y352, Syk S297 (ci). Quantitative data of (aii) Syk Y525/526, (bii) LAT Y191, (biii) PLCγ2 Y759, (cii) Syk Y352, and (ciii) Syk S297 are represented as means ± S.D from three technical experiments, with platelets from the same donor. ** p < 0.01, *** p < 0.001, # p < 0.0001, ns p > 0.05.