Highlights
-
•
Increasing evidence suggests that patients with cancer diagnoses may be particularly vulnerable to poor outcomes from COVID-19.
-
•
To our knowledge, this is the first such study to report on outcomes amongst a primarily Hispanic-American population of cancer patients with COVID-19.
-
•
Elevated ANC, AST, CRP, and LDH at time of admission were significantly associated with severe outcomes.
While the patients in our study received a variety of treatments, none were found to improve the occurrence of severe outcomes based on multivariate correlation analysis
Keywords: Coronavirus, Covid-19, Pandemic, Cancer, Malignancy
Abstract
We conducted a retrospective analysis of cancer patients who presented to the hospital with COVID-19 infection at a safety-net hospital in Los Angeles, California, from March 2020 to June 2020. From a list of 1,163 COVID-19+ adult patients, we selected the first 50 patients with malignancy for a preliminary analysis. There were 23 males (46.0%) and 27 females (54.0%); the median age was 60.5 years (IQR 47 – 72). Thirty-nine (78.0%) of the patients were Hispanic. The most prevalent cancers were genitourinary (14, 28.0%), hematologic (11, 22.0%), and gastrointestinal (10, 20.0%). Twenty-one (42.0%) patients had active disease at COVID-19 diagnosis, while 25 (50.0%) had no evidence of disease (NED), and 4 (8.0%) were unknown. Over 1 in 3 admitted patients experienced a “severe outcome,” which was defined as critical level care (14, 34.1%), use of vasopressors (9, 22.0%), intubation (8, 19.5%), or death (5, 12.2%). Patients with severe outcomes were found to have statistically higher values of absolute neutrophil count (p = 0.005), aspartate aminotransferase (p = 0.049), high-sensitivity C-reactive protein, (p = 0.001) and lactate dehydrogenase (p = 0.040) on admission. Overall survival (OS) was not statistically different between those with hematologic versus solid malignancy nor between those with active disease versus remission (both p>0.05). Thirteen (81.3%) of the 16 patients who had cancer treatment in 2020 experienced delays in cancer therapy. Additional cases are being evaluated as the pandemic continues with the goal of identifying areas for potential intervention to improve outcomes in this at-risk population.
Introduction
The first documented cases of COVID-19 originated in Wuhan, China in December 2019 [1]. The beta-coronavirus rapidly spread, resulting in the World Health Organization (WHO) declaring COVID-19 a “pandemic” on March 11, 2020 [2]. While it was shown early in the outbreak that patients with comorbid conditions were more likely to suffer severe disease from COVID-19 [3], there is less information as to how patients with cancer were affected by the novel virus.
Early data from China including limited numbers of patients with cancer suggested that a history of cancer or recent cancer-directed treatment was associated with more severe outcomes during COVID-19 infection [4, 5]. Subsequent studies among COVID-19 patients in New York City (NYC) demonstrated high rates of mortality in patients with cancer and COVID-19, with death rates ranging from 11- 28% [6], [7], [8]. Among the NYC population of COVID-19 patients, breast and genitourinary (GU) cancers were the most frequently described malignancies [6, 7]. In June 2020, the COVID-19 and Cancer Consortium (CCC19) registry database published data on 928 cancer patients infected with COVID-19 across the United States, Canada, and Spain with data collection occurring between March and May 2020. From this registry, breast and prostate cancer were the two most common malignancies. Meanwhile, risk factors associated with 30-day mortality included male gender, older age, presence of 2 or more comorbidities, former smoking status, poor performance status, and active cancer progressing on treatment [9]. Another multicenter study, the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT), published data on 200 patients with thoracic cancers (lung, mesothelioma, thymic and pulmonary neuroendocrine neoplasms) from 8 different countries (including Europe, USA, and China) in July 2020; the authors reported a death rate of 33% for patients with thoracic malignancies, with age greater than 65, current/former smoking, treatment with chemotherapy alone, and comorbidities associated with an increased risk of death in this group [10].
Given that cancer patients may face higher complications from COVID-19 infection, further research is needed to improve the management and treatment of patients with cancer during this pandemic. In this study, we aim to characterize COVID-19 infection in cancer patients at the largest public safety net hospital in Los Angeles with hopes that our findings will help influence surrounding oncological practices during this unprecedented time.
Materials + Methods
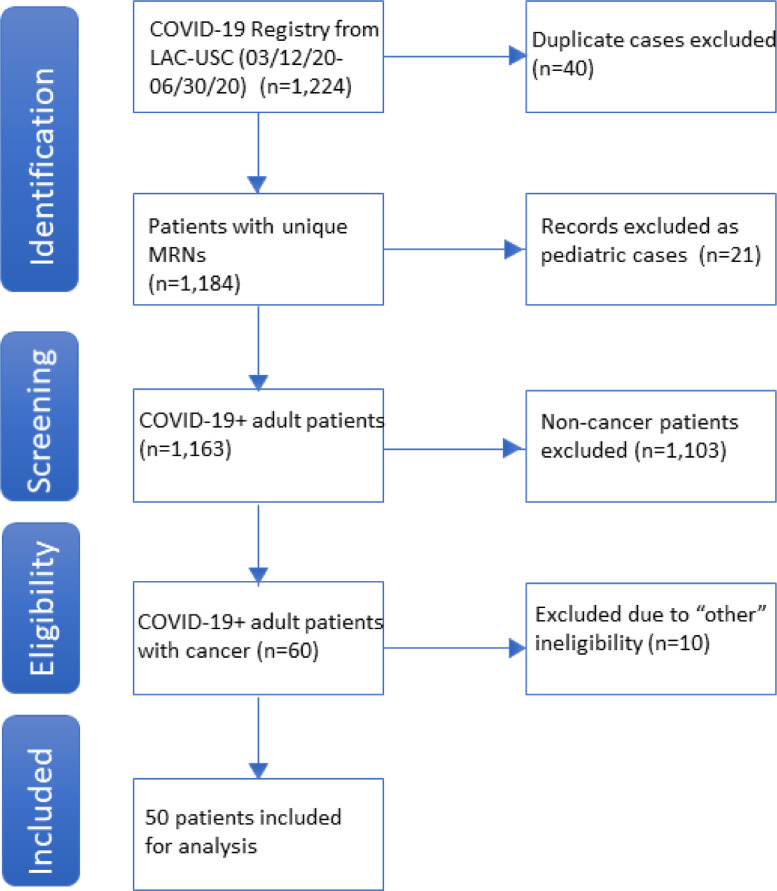
We performed a retrospective review of the first 50 cancer patients who were admitted to the Los Angeles County + University of Southern California Medical Center (LAC-USC) or who presented to the LAC-USC ED, and who tested positive for SARS-CoV-2. SARS-CoV-2 positivity was based on nasopharyngeal swab using the Xpert Xpress SARS-CoV-2 assay (Cepheid). Similar to the eligibility criteria used by Kuderer et al., we included all patients at least 18 years of age with a positive SARS-CoV-2 test and a current or prior diagnosis of hematologic or solid tumor malignancy; non-melanoma skin cancers, in-situ neoplasm and precursor hematologic neoplasms were excluded (Fig. 1 ) [9]. The cutoff of 50 patients was chosen in order to expedite a preliminary analysis that would allow rapid dissemination of meaningful data on this vulnerable population in real time during the ongoing pandemic. Chart review for these 50 patients was conducted from May 19, 2020 through July 7, 2020 and included patients who tested positive from March 12, 2020 to June 30, 2020. This study was reviewed and approved by the Institutional Review Board at the University of Southern California.
Fig. 1.
PRISMA Diagram Depicting Identification and Selection of Eligible Patients.
Data collection
Clinical data, including patient demographics (age, gender, race, comorbidities), cancer history (histology, stage, treatment, active [measurable] versus remission), clinical course during COVID-19 infection, and other relevant variables were abstracted from the electronic medical record. Due to differences in staging systems across cancer types, we categorized cancer stage as either “limited” or “advanced”, based on the need for systemic cancer therapy (i.e. “limited” stage cancers did not require systemic therapy, while “advanced” cancers did). Laboratory studies of interest were collected at the time of admission, and also throughout the hospital course for all patients, when available.
Descriptive statistics were reported as median with interquartile ranges. Categorical variables were reported as total number and percentages. Prism v. 8.4.2 (GraphPad Software, LLC) was utilized for advanced statistical analysis. Statistical significance was defined as p<0.05. No imputation was made for missing data. Kaplan-Meier survival curve analyses were performed using log-rank (Mantel-Cox) tests. Correlation tests were used to calculate Pearson correlation coefficient (Pearson r) values and multiple linear regressions utilized least squares. Overall survival was calculated from the date of hospital presentation or ED admission.
Endpoints
The primary endpoint of this study was to evaluate the occurrence of severe outcomes due to COVID-19 infection among patients with current or prior malignancy. Severe outcomes were defined in this study as either: admission to an intensive care unit, the use of vasopressors, intubation, or death.
Results
Demographics
The 50 patients analyzed consisted of 23 males (46.0%) and 27 females (54.0%) (Table 1 ). The median age at COVID-19 diagnosis was 60.5 years (IQR 47–72). The majority of patients (78.0%) identified as Hispanic. Among all 50 patients, 76.0% had at least 1 comorbidity, including 52.0% with hypertension and 38.0% with diabetes.
Table 1.
Cohort Demographics and Cancer History for the First 50 Cancer Patients with Confirmed COVID-19 Infection at LAC-USC.
| (n = 50) | |
|---|---|
| Age at Diagnosis (median, IQR) | 60.5 (47 – 72) |
| Gender | |
| Male | 23 (46.0%) |
| Female | 27 (54.0%) |
| Race | |
| Hispanic/Latino | 39 (78.0%) |
| Asian/ Pacific Islander | 4 (8.0%) |
| African American | 2 (4.0%) |
| Non-Hispanic White/Caucasian | 1 (2.0%) |
| Other | 4 (8.0%) |
| Other Comorbidities: | |
| 0 |
12 (24.0%) |
| 1 | 11 (22.0%) |
| 2 | 15 (30.0%) |
| 3 | 7 (14.0%) |
| 4+ | 5 (10.0%) |
| Cancer Diagnoses: | |
| Genitourinary | 14 (28.0%) |
| Hematologic | 11 (22.0%) |
| Gastrointestinal | 10 (20.0%) |
| Gynecologic | 5 (10.0%) |
| Breast | 3 (6.0%) |
| Skin/soft tissue | 3 (6.0%) |
| Lung/thoracic | 1 (2.0%) |
| Other | 7 (14.0%) |
| Number of patients with more than 1 cancer diagnosis | 4 (8.0%) |
| Highest Cancer Stage: | |
| Limited |
20 (40.0%) |
| Advanced | 27 (54.0%) |
| Unknown | 3 (6.0%) |
| Prior Treatment History: | |
| Surgery |
31 (62.0%) |
| Radiation | 09 (18.0%) |
| Systemic Therapy | 33 (66.0%) |
| Cytotoxic Therapy | 20 (40.0%) |
| Targeted Therapy | 8 (16.0%) |
| Endocrine/Hormonal Therapy | 5 (10.0%) |
| Immunotherapy | 0 (0.0%) |
| None | 3 (6.0%) |
| Other | 5 (10.0%) |
| Current Cancer Status: | |
| Active |
21 (42.0%) |
| Remission / No evidence of disease | 25 (50.0%) |
| Unknown | 4 (08.0%) |
Malignancy history
The most prevalent types of cancer in our cohort included genitourinary cancers (28.0%), hematologic cancers (22.0%), and gastrointestinal cancers (20.0%) (Table 1). Four patients (8.0%) reported having a history of two separate primary malignancies. Twenty-one patients (42.0%) had active disease at COVID-19 diagnosis, while 25 patients (50.0%) were considered to be in remission and 4 (8.0%) had an unknown cancer status.
Of note, we found that 81.3% of the 16 patients who had any type of cancer treatment in 2020 experienced delays in cancer therapy secondary to the impact of the COVID-19 pandemic on the healthcare system or due to their own COVID-19+ status. The most frequent therapy delayed was chemotherapy, affecting 62.5% of patients receiving therapy in 2020.
Hospital course and outcomes for admitted patients (n = 41)
Forty-one of the 50 patients (82.0%) included in this analysis were ultimately admitted, including 10 patients who underwent any cancer-directed therapy within 30 days prior to a positive COVID-19 test. Among these, 85.4% were started on some type of anticoagulation (prophylactic, therapeutic, or both), and 4.9% of patients developed thromboses (Table 2 ). In addition, 63.4% were administered antibiotics and 26.8% received systemic steroids while admitted.
Table 2.
Clinical Outcomes among Cancer Patients Hospitalized during COVID-19 Infection.
| (n = 41) | |
|---|---|
| Developed Fever in Hospital? | |
| Yes |
23 (56.1%) |
| No | 18 (43.9%) |
| Findings on initial chest x-ray? n = 37 | |
| Yes |
28 (75.7%) |
| No | 9 (24.3%) |
| Therapies Administered | |
| Azithromycin | 16 (39.0%) |
| Convalescent Plasma | 7 (17.1%) |
| Redemsevir | 5 (12.2%) |
| Hydoxychloroquine/ Chloroquine | 2 (4.9%) |
| Toculizumab | 1 (2.4%) |
| Systemic Steroids | 11 (26.8%) |
| Other Supportive Measures | |
| Transfusions | 9 (22.0%) |
| Vasopressors | 9 (22.0%) |
| Antibiotics | 26 (63.4%) |
| Prophylactic anticoagulation | 31 (75.6%) |
| Therapeutic anticoagulation | 12 (29.3%) |
| Admitted to a critical care team | 14 (34.1%) |
| Patients requiring mechanical ventilation | 8 (19.5%) |
| Length of Intubation in days (median, IQR) | 16 (11.75 – 22 0.5) |
| Death | |
| Yes |
5 (12.2%) |
| No | 36 (87.8%) |
Fourteen (34.1%) of the admitted patients fit the predefined category of experiencing a severe outcome requiring critical level care. All 14 were treated by the critical care team. In addition, 22.0% of admitted patients required vasopressors and 19.5% required intubation with a median length of intubation of 16 days (IQR 11.75–22.5). Two (4.9%) patients required a tracheostomy. Five patients (12.2%) ultimately died from complications related to COVID-19; two of these patients had active cancer, while three were in remission. Of note, only 1 out of the 10 patients who had recent cancer therapy (within 30 days prior to COVID-19 diagnosis) experienced a severe outcome, which was admission to the ICU.
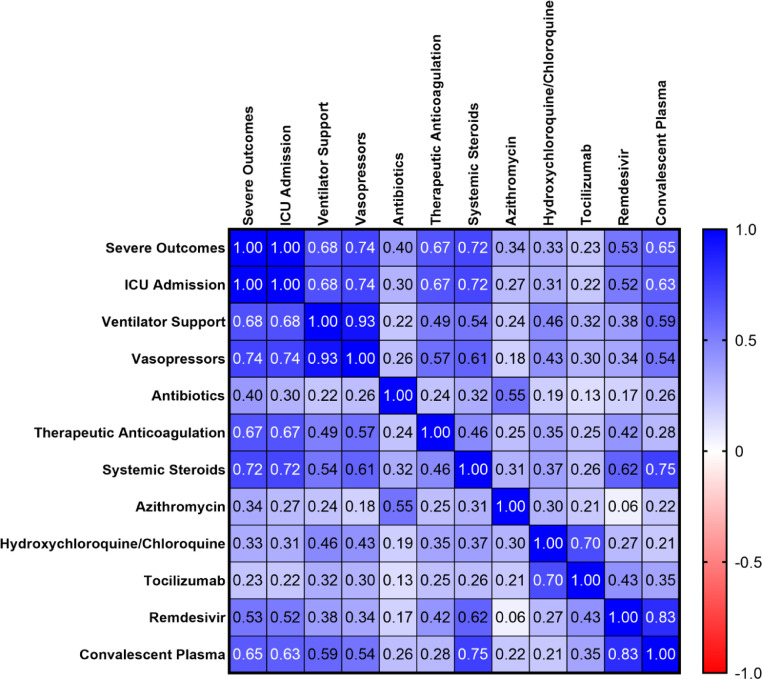
In order to assess the impact of different therapies on severe outcomes from COVID-19 infection, we then performed multivariate correlation analysis. Amongst all therapies analyzed however, none were associated with a benefit in terms of severe outcomes (Fig. 2 ). In fact, all were significantly associated with severe outcomes, with the exception of tocilizumab, which had a non-significant trend towards severe outcomes (p = 0.11), but this was only administered to 3 patients in total. Confounding by indication cannot be excluded.
Fig. 2.
Correlation of Individual Treatments to Likelihood of Severe Outcomes Denoted by Pearson r Coefficient.
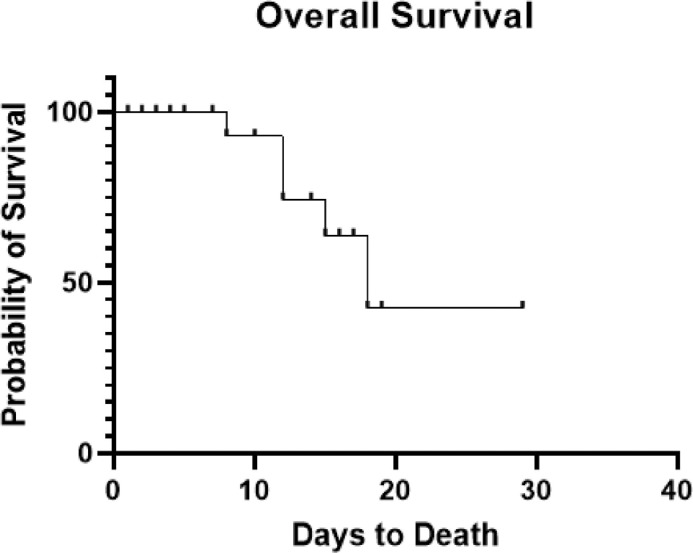
Overall survival was not significantly different between those patients with hematological and non-hematological malignancies (p = 0.57). Additionally, overall survival was not significantly different between patients with active cancer versus those in remission (p = 0.55). A survival curve based on all patients can be seen in Fig. 3 .
Fig. 3.
Kaplan-Meier Survival Curve for All Patients with Cancer Diagnosis and Concurrent COVID-19 Infection. Patients were censored at the time of discharge or death, as denoted by ticks on the curve. Patients who were not admitted or were discharged on the same day of admission were excluded from this analysis.
Laboratory findings
Serology studies at the time of admission revealed multiple abnormalities consistent with other published studies of COVID-19 patients. Lymphopenia was present in 72.3% of patients. Inflammatory markers were elevated in greater than 70.0% of those tested for ferritin, high sensitivity C-reactive protein (CRP), lactate dehydrogenase (LDH), and procalcitonin. Markers of coagulopathy were also frequently present, with d-dimer and fibrinogen elevated in 90.3% and 60.0% of patients, respectively.
When comparing blood-based biomarkers among patients with active cancer against those who were disease free/in remission, patients who were cancer free were found to have significantly higher white blood cell counts (WBC, p = 0.010), absolute neutrophil counts (ANC, p = 0.032), and platelets (PLT, p = 0.047). No other laboratory studies were significantly different between the groups. In addition, there were no significant differences in admission laboratory values when comparing patients with hematological malignancy to those with solid tumors.
We then compared admission laboratory values between patients with severe outcomes (admission to intensive care unit, intubation, pressor support or death) and those without. ANC (p = 0.005), aspartate aminotransferase (AST) (p = 0.049), CRP (p = 0.001) and LDH (p = 0.040) were all significantly higher in patients with severe outcomes. d-dimer values were numerically higher in patients with severe outcomes but this did not reach statistical significance (p = 0.053). No other laboratory studies significantly differed between patients with and without severe outcomes.
For patients who had repeat blood draws throughout hospitalization course, maximum and minimum values were recorded on laboratory values of interest. The majority of patients were found to have anemia, thrombocytopenia, or lymphopenia at some time point during their hospital course. Elevations in transaminases were seen in approximately 60–70% of patients who had these values trended. Greater than 93% of patients were found to have elevations in inflammatory markers at least once throughout their hospital course. An elevated dimer was found in 100% of patients who had their dimer levels trended. T-tests were not conducted on maximum or minimum values as the total number of patients with repeat laboratory values was much lower than the number of patients who had bloodwork on admission.
Discussion
The impact of the COVID-19 pandemic has differentially affected unique patient populations across distinct geographic locations at different periods of time. Increasing evidence suggests that patients with cancer diagnoses may be particularly vulnerable to poor outcomes from this infection. Our goal was to evaluate outcomes among cancer patients with COVID-19 infection at LAC-USC, a public county hospital in Los Angeles that serves a primarily low-income population.
There are many unique characteristics of our patient population. To our knowledge, this is the first such study to report on outcomes amongst a primarily Hispanic population of cancer patients with COVID-19. Second, as our inclusion criteria required that patients be evaluated at an ED or admitted, this likely skewed our population with more clinically serious COVID-19 infection, whereas some studies included all patients, or only symptomatic patients. Nevertheless, we did find that our clinical outcomes were largely consistent with other similar studies.
To help describe the clinical impact of COVID-19 infection on cancer patients in a more comprehensive manner, we collected data on ICU admissions, intubation, use of vasopressors and death; we found that these severe outcomes occurred in 28.0% of all patients. Unlike other studies, which reported that 8–12% of patients were admitted to an ICU [7], [8], [9], [10], our ICU admission rate was 28%, although we again emphasize the fact that we included only patients who required either ED evaluation or inpatient management in our cohort. Meanwhile, the rate of intubation (16%) was more consistent with the rate of 6–20% reported by others [6], [7], [8], [9], [10]. We reported the use of vasopressor support among 18% of our entire patient population. While the use of vasopressor support has not been well documented in other studies, in the TERAVOLT study, 5.1% of patients were reported to have developed sepsis during COVID-19 infection [10]. The overall death rate of 10.0% of all patients in our study is similar to the rate of 11.1–13.0% found in the CCC-19 study and others [6, 8, 9] but lower than the 33% reported from TERAVOLT and 28% reported by Mehta et al. [7, 10]. The lower rate of death and other complications at our institution may be explained by the fact that Los Angeles experienced the pandemic chronologically later than China, Europe and New York City. As such, our hospital was able to utilize initial knowledge from other institutions to guide management (e.g. aggressive anticoagulation, proning) as standard practices. In addition, Los Angeles experienced a more gradual upstroke in infection rates, compared to other geographic areas, and thus our health care facility was not routinely faced with such extreme shortages of medical staffing and other critical interventions, as compared to other locations. Furthermore, we note that our patient population had a low percentage of patients with thoracic cancers, which others have reported to portend a worse prognosis (up to 33% death rate) [10], in association with ARDS.
Multiple studies have found that clinical outcomes are worse for COVID-19 patients with hematological malignancy compared to solid tumors [7], and yet we did not observe this difference in our population. We speculate that this may be due to heterogeneity between study populations and our small sample size; specifically, we note that among our small cohort of 11 patients with hematologic malignancy, only one patient had acute B-cell leukemia, while the remaining patients had either chronic leukemia, lymphoma or myeloma, with more than half of the patients being in remission. Similarly, the study by Brar et al., which featured primarily chronic leukemia patients, also did not reveal a difference between hematologic and solid tumor malignancies[11].
The CCC19 study, as well as that by Mehta et al., both suggest that active cancer is associated with worse clinical outcomes to COVID-19, as compared to non-active cancer [7, 9], but this too, was not statistically significant in our patient cohort (p = 0.55). We caution that this lack of association was likely influenced by the relatively small sample size in our preliminary analysis and this will have to be further evaluated during later analysis when more patients are included. In addition to the question of how active cancer versus remission affects COVID-19 outcomes, there is also the issue of active cancer treatment, and its impact. There are at least 4 studies which suggest that active cancer therapy with either surgery or cytotoxic chemotherapy does not lead to worse outcomes [[7], [8], [9], 11]. On the other hand, the TERAVOLT study did find an association between active chemotherapy use and death from COVID-19, while the study by Robilotti and colleagues from Memorial Sloan Kettering also noted that use of immune checkpoint inhibitors was associated with severe outcomes as well [8, 10]. Future studies should establish strict criteria to define “active cancer” and measure the effect of specific therapies, with larger numbers of patients, to help determine which subgroups of cancer patients may be at greatest risk from COVID-19 infection.
Throughout the course of the COVID-19 pandemic, improvements in supportive care have led to better clinical outcomes. While the patients in our study received a variety of treatments (including convalescent plasma, hydroxychloroquine, therapeutic anticoagulation and azithromycin), none were found to improve the occurrence of severe outcomes based on multivariate correlation analysis. However, we caution against overinterpretation of these findings, as our sample size is limited, and without an appropriate comparison group. Aside from severe outcomes, our study did produce a number of interesting clinical findings that warrant mention. In comparison to other studies which have reported venous thromboembolism in up to 20% of COVID-19+ patients, in our study a thrombotic event was found in only 2 (4.9%)admitted cancer patients [12]. This is surprising as patients with malignancy are generally hypercoagulable, due to multiple factors, including the malignancy itself, cancer therapies (e.g. surgery, chemotherapy) and other complications, with some estimates that up to 20% of cancer patients will experience a thromboembolic event [13]. The lower frequency of thromboembolic events seen in our population may be due to aggressive use of anticoagulation (either prophylactic or therapeutic), based on hospital distributed guidelines on anticoagulating COVID-19+ patients.
In addition to coagulopathy, cancer patients are often at risk of immunosuppression, either from cancer-directed therapies or from the underlying disease itself. The RECOVERY trial, published July 17, 2020, reported a lower incidence of death in a general patient population who was requiring supplementary oxygen when treated with dexamethasone; this benefit was attributed to the ability of dexamethasone to reverse the inflammatory effects of COVID-19 infection [14]. The effects of steroids among cancer patients with COVID-19 has not been well studied. In our admitted population, only 26.8% of patients received systemic steroids, but this was not associated with any clinical impact. Similarly, other studies conducted before the RECOVERY study were unlikely to employ regular use of steroids, which also may have affected outcomes compared to other groups. The impact of systemic steroids among cancer patients infected with COVID-19 must be further evaluated, particularly in the context of immune checkpoint inhibitors.
In our analysis, elevated ANC, AST, CRP, and LDH at time of admission were significantly associated with severe outcomes; elevated dimer showed a trend towards worse outcomes but did not reach statistical significance (p = 0.053). Similar studies have shown that elevations in LDH and dimer have been associated with severe respiratory illness and increased mortality in this patient population [7, 8]. Additionally, elevated ANC has also been associated with death in at least one other study [7]. We propose further investigation of these biomarkers to help stratify patients at highest risk of severe outcomes, where more aggressive interventions (e.g. higher nursing ratio for closer monitoring, aggressive use of therapeutics) may help improve clinical outcomes during COVID-19 infection.
Of particular concern to the authors was the finding that the majority (81.3%) of patients who had any type of cancer treatment in 2020 experienced delays in treatment secondary to the impact of the COVID-19 pandemic on the healthcare system or secondary to their own COVID-19+ status. Ongoing administrative changes at the hospital level, as well as city, state and national level, have impacted delivery of care for cancer patients during the pandemic, but it does appear that these delays will only improve as precautionary measures are more easily and readily instituted. Large modeling studies suggest that delays in cancer-directed care, due to COVID-19, may have significant impact on cancer outcomes, including additional deaths and years of life lost due to cancer, thus pointing towards a critical negative impact on health systems in the future [15].
In conclusion, we present one of the first analyses of cancer patients with COVID-19 infection from Los Angeles, including a diverse patient population treated at a safety-net hospital. Given the later occurrence of the pandemic in the Western United States, our institution was able to implement important clinical measures. We present our initial data and preliminary hypotheses in hopes that this will lead to further clinical improvements during this unprecedented time. As mentioned above, we acknowledge several limitations to our study, including the retrospective design, and that our population was limited to a single institution, with a small sample size. Conversely, we must emphasize that our population does include a diverse population (predominantly Hispanic-American) of cancer patients, that to date has not been well represented. Our goal is to continue to develop a more robust database throughout the ongoing course of the COVID-19 pandemic to further validate these findings and identify other targets for clinical intervention going forward.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Gino In, MD: Advisory boards/Consulting: Sanofi, BMS, Novartis. Speaker: Merck. Clinical Trials: Genentech, Idera, Regeneron, Iovance.
Acknowledgement
This project was supported by the University of Southern California NCI Cancer Center Support Grant P30 CA014089, as well as the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health, grants (UL1TR001855 and UL1TR000130).
References
- 1.Zhu N. A Novel Coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, Director-General's opening remarks at the media briefing on COVID-19. 2020.
- 3.Guan W.J. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang, L., et al., Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol, 2020. [DOI] [PMC free article] [PubMed]
- 6.Miyashita H. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta V. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robilotti E.V. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuderer N.M. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garassino M.C. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brar G. COVID-19 Severity and Outcomes in Patients With Cancer: a Matched Cohort Study. J Clin Oncol. 2020 doi: 10.1200/JCO.20.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland D.E., Weitz I.C., Liebman H.A. Thromboembolic complications of cancer: epidemiology, pathogenesis, diagnosis, and treatment. Am J Hematol. 2003;72(1):43–52. doi: 10.1002/ajh.10263. [DOI] [PubMed] [Google Scholar]
- 14.Horby, P., et al., Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med, 2020. [DOI] [PMC free article] [PubMed]
- 15.Maringe C. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]