Figure 1. ASK-interacting protein 1 (ASK1) activation during lipotoxicity:
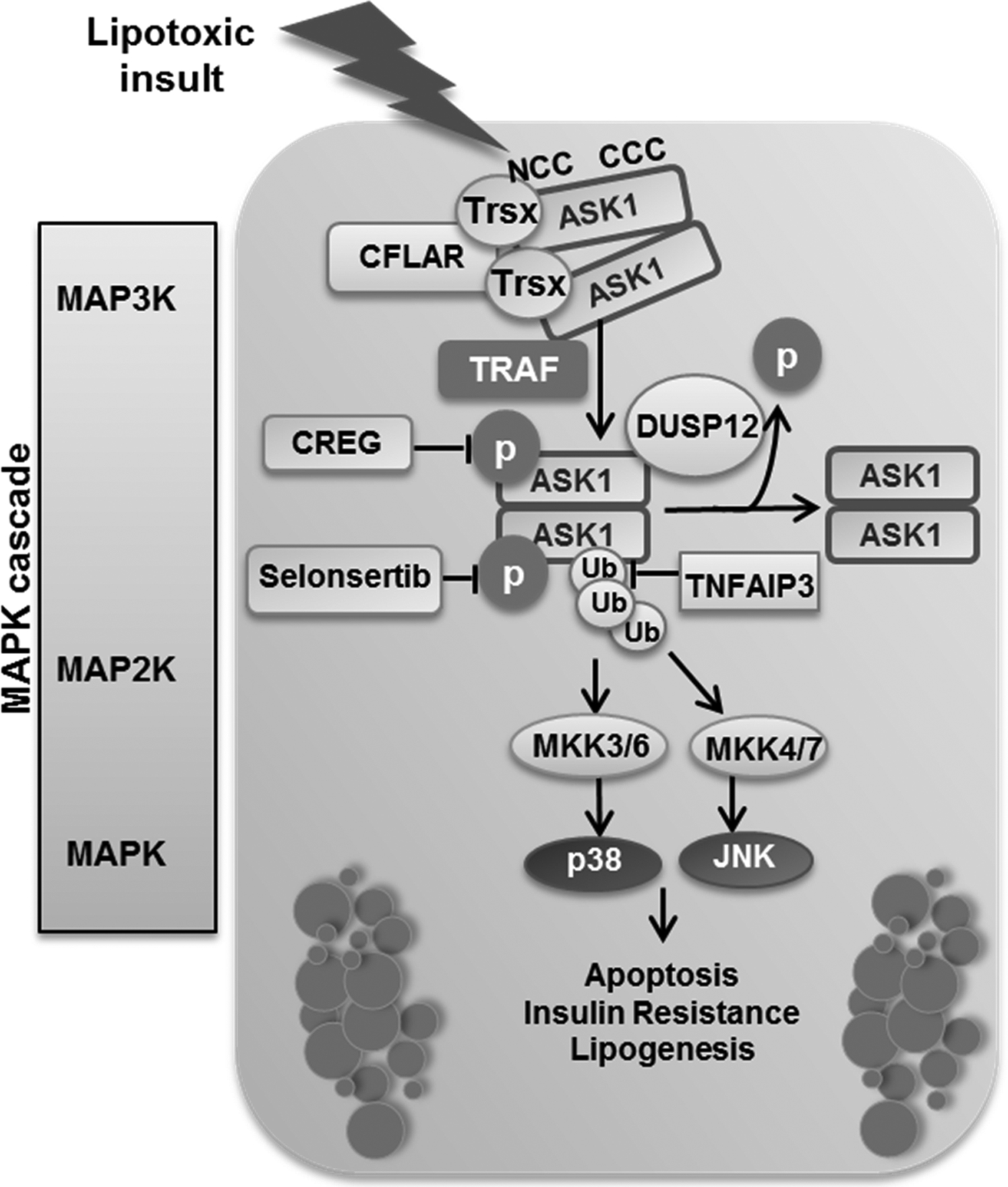
ASK1 forms a high-molecular-mass complex through its carboxy‑terminal coiled-coil (CCC) domain. The thioredoxin (Trx) binding site is located near the amino‑terminal coiled-coil (NCC) domain and keeps ASK1 inactive. Under conditions of stress, Trx dissociates from ASK1 and tumor necrosis factor (TNF) receptor-associated factor (TRAF) molecules are recruited and induce oligomerization of ASK1, resulting in its autophosphorylation and activation. CASP8 and FADD-like apoptosis regulator (CFLAR) target the N‑terminal-mediated dimerization of ASK1 and blocks its activation. Dual specificity phosphatase (DUSP) 12 dephosphorylates ASK1, which leads to its inactivation. Selonsertib binds to the catalytic kinase domain of ASK1 in an ATP-competitive manner and inhibits its activity. Cellular repressor of E1A-stimulated genes (CREG) interacts directly with ASK1 and inhibits its phosphorylation. The deubiquitinase TNF alpha-induced protein 3 (TNFAP3) deubiquitinates and suppresses ASK1. ASK1 activation triggers a mitogen activated protein kinase (MAPK) signaling cascade leading to c-Jun N‑terminal kinase (JNK) and p38 activation, which results in insulin resistance, lipogenesis, and apoptosis. MKK, mitogen-activated protein (MAP) kinase kinase; P, phosphate group; Ub, ubiquitin.
