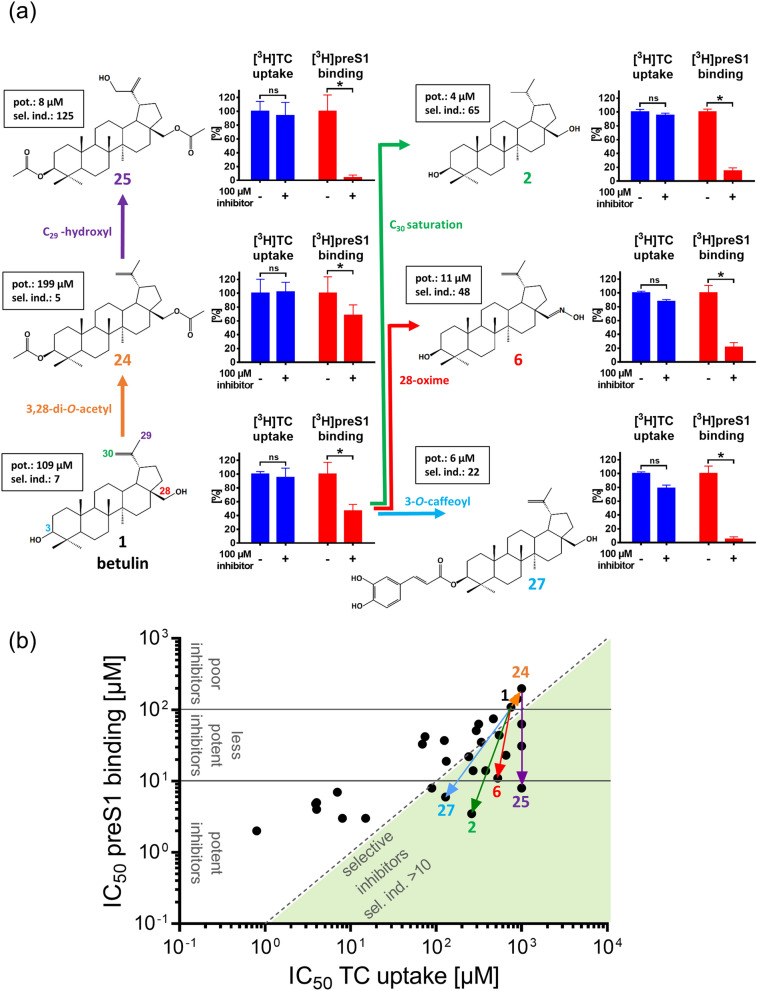
Figure 3.
Structure–activity relationships of betulin derivatives for [3H]TC transport inhibition and [3H]preS1 binding inhibition. Chemical modifications at the lupane skeleton of betulin revealed clear structure–activity relationships for the potency (pot., IC50 of [3H]preS1 binding inhibition) and virus selectivity index (sel. ind., ratio IC50 [3H]TC transport inhibition / IC50 [3H]preS1 binding inhibition). (a) 3,28-di-O-acetylation (shown in orange), additional C29-hydroxylation (shown in purple), 3-O-modification with caffeoyl (shown in light-blue), 28-oxime generation (shown in red), and C30 saturation (shown in green) of the betulin core structure improve the potency and/or selectivity index of the respective compounds. The respective inhibition pattern are illustrated by bar graphs for [3H]TC uptake (shown in dark-blue) and [3H]preS1 peptide binding (shown in red), respectively, in the presence or absence of a fix concentration (100 µM) of the respective betulin derivative. Graphs show means ± SD of quadruplicate determinations. *Significantly different with p < 0.01 (two-way ANOVA with Sidak's multiple comparison test); ns, not significant. (b) The diagram shows all IC50 values for [3H]TC uptake inhibition and [3H]preS1 binding inhibition of the respective compounds. Effects (IC50 shifts) of the structural modifications are illustrated by arrows, starting from the core structure betulin (compound 1). Compounds can be divided into three groups regarding their potency to inhibit [3H]preS1 binding: potent inhibitors (IC50 ≤ 10 µM), less potent inhibitors (10 µM < IC50 < 100 µM) and poor inhibitors (IC50 ≥ 100 µM). Compounds with a selectivity index of > 10 are classified as selective inhibitors.