Figure 3.
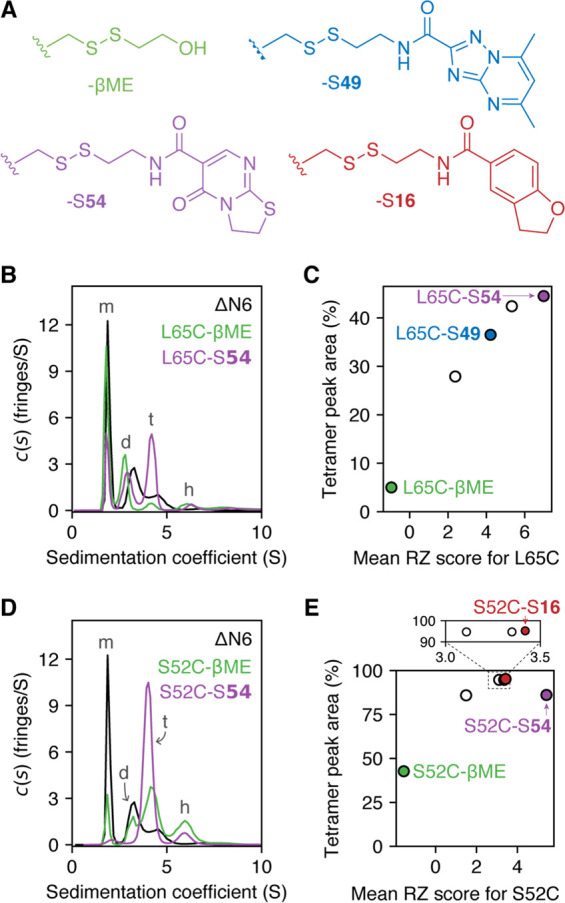
(A) Examples of fragments used to form the S52C– and L65C–fragment adducts studied by SV-AUC. (B–E) SV-AUC data collected for L65C–fragment adducts (B, C) or S52C–fragment adducts (D, E). Experiments were performed with 150 μM protein in 25 mM sodium phosphate at pH 6.2, 25 °C. Peaks in the c(s) distributions were assigned to monomer (m), dimer (d), tetramer (t), or hexamer (h), based on previous studies37 and predicted sedimentation coefficients for these oligomers (calculated using the Svedberg equation48). Tetramer peak areas for a range of L65C–fragment adducts were found to correlate with the RZ score of the attached fragment (C). The relationship between fragment RZ score and tetramer peak area for S52C–fragment adducts was less clear, and other properties of the fragments may play a role in modulating tetramer populations (E) (see Figure S14).
