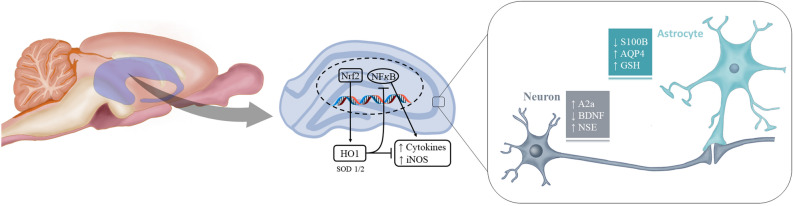
Figure 4.
Schematic illustration of some cellular targets of ZIKV in neural cells. Our data reinforce the strong neurotropism of ZIKV, which was able to readily increase the expression and/or release of pro-inflammatory mediators, such as cytokines and iNOS. Inflammatory response is mainly coordinated by NFκB. In contrast, Nrf2 and its transcriptional products, such as HO1, are important regulators of adaptive responses to cellular stresses. HO1 is able to counteract inflammatory response and NFκB transcription activity. However, both Nrf2 and HO1 were downregulated by ZIKV exposure. More specific neuronal and astroglial ZIKV-induced effects could also be observed. A decrease in BDNF release, an increase in NSE and in A2a receptor gene expression can be mainly attributed to neurons (although A2a can be also expressed by astrocytes and microglia). Moreover, a decrease in S100B release, as well as an increase in mRNA levels of AQP4 and in GSH content can indicate an acute ZIKV-induced glial commitment in the hippocampus of adult rats.