Figure 1.
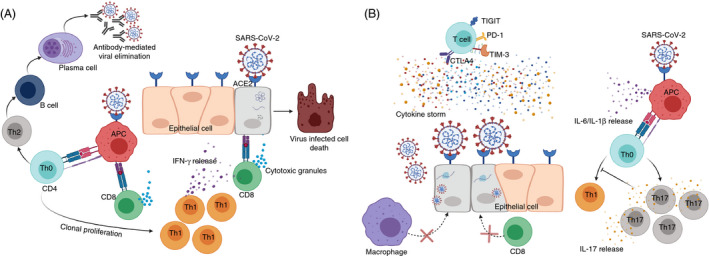
T‐cell responses against SARS‐CoV‐2. SARS‐CoV‐2 recognizes cells expressing ACE2 receptor including epithelial cells and macrophages. In normal immune environment, infected epithelial cells degrade viral particles and present them to cytotoxic CD8+ T cells (CTLs). CTLs detect viral protein through classical TCR‐MHC I interaction, release cytotoxic granules, including granzyme B and perforin, and eliminate infected cells. Additionally, macrophages detect SARS‐CoV‐2 via ACE2 receptor and present the virus‐derived peptides to CD4+ T cells (Th0) via TCR‐MHC II interaction. Once exposed to antigen, Th0 cells polarize primarily towards Th1, leading to the release of IFN‐γ to eliminate the virus, and Th2 to trigger humoral‐mediated immune responses and antibody secretion against SARS‐CoV‐2 virus (A). In incompetent immune environment, SARS‐CoV‐2 recognizes epithelial cells or macrophages via ACE2 receptor. Viral RNA will replicate by hijacking the host transcriptional machinery. These viral progenies will infect multiple cells leading to tissue damage and further lethal complications. In these circumstances, CD4+ and CD8+ T cells fail to provide adequate cell/humoral‐mediated immune responses to eliminate viral‐infected cells. On the other hand, Th0 cells are primed towards Th17 phenotype, resulting in the inhibition of Th1‐mediated immune responses (B). In COVID‐19, T cells could be exhausted and could overexpress exhaustion markers including PD‐1, CTLA‐4, TIM‐3 and TIGIT through unknown mechanisms . In severe COVID‐19 cases, the production of cytokines, including IL‐1β, IL‐6, IL‐2, IL‐10 and TNF‐α, is increased leading to the generation of cytokine storm, which induces further unfavourable outcomes and may eventually lead to lymphopenia (B).
