Abstract
Fungal cell walls are composed of a polysaccharide network that serves as a scaffold in which different glycoproteins are embedded. Investigation of fungal cell walls, besides simple identification and characterization of the main cell wall building blocks, covers the pathways and regulations of synthesis of each individual component of the wall and biochemical reactions by which they are cross-linked and remodeled in response to different growth phase and environmental signals. In this review, a survey of composition and organization of so far identified and characterized cell wall components of different yeast genera including Saccharomyces, Candida, Kluyveromyces, Yarrowia, and Schizosaccharomyces are presented with the focus on their cell wall proteomes.
Keywords: yeast cell wall, cell wall proteome, glucan, mannan, glucanases, transglycosylases
1. Introduction
Fungal cell walls are composed of glucans (β-1,3-, β-1,6-, β-1,4- and α-1,3-glucans), chitin, and glycoproteins. The basic component of the cell wall moiety in almost all fungal cell walls is the β-1,3-glucan. The composition of the N-linked and O-linked oligosaccharides in cell wall glycoproteins vary, with Saccharomyces cerevisiae and Candida albicans having high mannan structures and Sz. pombe having galactomannans. Schweigkofler et al. [1] analyzed monosaccharide patterns in the cell wall of different fungi and found three main types: (a) the glucose, mannose type; (b) the glucose, mannose, galactose type and (c) the glucose, mannose, galactose, rhamnose type. The glucose, mannose type predominated and it was found in 51 species of the genera Saccharomyces, Pichia, Candida, Debaryomyces, and Kluyveromyces, with very different mannose proportions ranging from 22% to 75% [1]. Glucose, mannose, galactose type was found in 26 strains within the genera Pichia, Candida, Arxula, Debaryomyces, and Yarrowia [1]. The proportion of glucose ranged from 28% to 65%, and the proportion of mannose from 18% to 56%. In almost all strains galactose was the least present, ranging from 2% to 27%. The same glucose, mannose, galactose pattern was found in Sz. pombe [1]. It is not clear what additional physico-chemical or structural properties have been acquired by the incorporation of galactose in the cell wall but it should be noted that it requires additional enzymes. Glucans, chitin, and glycoproteins are covalently cross-linked by a dynamic process that occurs extracellularly. All fungi contain a similar collection of the cross-linking enzymes capable of creating links among glucan, chitin, and glycoprotein molecules. Hydrolases and transferases are the primary contributors to the cell wall dynamics. The yeast genomes contain a number of genes coding for glucanases and chitinases, often having overlapping functions [2]. The glycosyltransferases have both synthetic and lytic activities, enabling them to cut molecules and then attach them back, building polymers in an environment in which no external source of energy is present. In order to achieve suitable elasticity to allow the growth of the cell wall and budding of cells, the activities of these enzymes must be strictly controlled. Cell wall proteins without known enzymatic activity are considered to have structural functions, to play roles in cell adhesion and mating, or to connect them to other cell wall constituents [3]. Genetic redundancy that exists among many cell-wall associated proteins additionally complicate the determination of the functions of different cell wall proteins. Accordingly, disruptions of single genes hardly ever result in a clear mutant phenotype. Sometimes, phenotypes are detected after the deletion of multiple genes of the same family. In this paper, an overview of enzymes and enzymatic activities that synthesize the carbohydrate part of cell walls of different yeasts is presented. Differences in the presence and type of these enzymes result in different compositions of the carbohydrate moieties of different yeast species. Furthermore, the findings on the composition of the protein part of the cell wall of different yeasts are summarized. The proteins are divided into three basic groups: the group of proteins that are enzymatically active, putatively structural wall proteins, and adhesins. Finally, an overview of current knowledge on wall proteomes of various yeasts is given.
2. Composition and Synthesis of Carbohydrate Parts of Yeast Cell Walls
2.1. Variations in the Composition of Carbohydrate Parts of Cell Walls of Different Yeasts
The S. cerevisiae cell wall consists largely of β-1,3-glucan (50–55%) with lesser amounts of β-1,6-glucan (10–15%) [4]. The wall also contains a small amount of chitin (1–2%). Most of the chitin is found in bud scars formed during the separation of mother and daughter cells during budding but a small amount is also found in lateral cell walls [4]. The β-1,6-glucan is attached to the non-reducing ends of β-1,3-glucan chains participating in the formation of the cell wall matrix [4,5]. The cell wall proteins contain N-linked outer chain mannan with up to 200 mannose residues and short O-linked mannan chains of up to 5 mannose units. GPI (glycosylphosphatidylinositol) anchored proteins are attached to β-1,6-glucans that are connected with the oligosaccharide part of the GPI anchor [6]. Melanin, α-1,3-glucan, and galactomannans are absent from the S. cerevisiae cell wall. The yeast wall is organized so that the outer surface of the wall contains large amounts of mannan, i.e., mannoproteins, while glucans and chitin are more concentrated in the layer of the cell wall adjacent to the plasma membrane.
In most aspects, the C. albicans cell wall resembles the one of S. cerevisiae [7,8]. The major constituents are β-1,3-glucan (30–39%) and β-1,6-glucan (43–53%), with a small amount of chitin (2–6%) [7]. As in S. cerevisiae, the β-1,6-glucan is attached to the β-1,3-glucan chains. The wall lacks α-1,3-glucan and galactomannans. During host infection, the C. albicans wall contains melanin, which is thought to help strengthen the wall. C. albicans synthesizes N- and O-linked mannans attached to the cell wall glycoproteins.
The carbohydrate part of the Sz. pombe cell wall is composed of 42% β-1,3-glucan, 2% β-1,6-glucan, 9–14% α-galactomannan, and 18–28% α-1,3-glucan [9]. The cell wall consists of three layers [9] and lacks chitin [10,11]. Furthermore, instead of the branched β-1,6-glucan, it contains short polymers termed diglucan (2%) comprising a backbone of β-1,6-linked glucoses, 75% of which carry β-1,3-linked glucoses [11]. α-Glucan is probably synthesized at the cell membrane, according to the localization of synthase Mok1/Ags1p at the cell membrane [12,13]. Humbel et al. [14] investigated the distribution of β-1,6-glucan, β-1,6-branched β-1,3-glucan, and linear β-1,3-glucan in the cell wall of Sz. pombe and found linear β-1,3-glucan exclusively localized in the primary septum of dividing cells. Both β-1,6-branched β-1,3-glucan and β-1,6-glucan form a less dense, central layer of the wall, with β-1,6-glucan located in the outer part of this layer, close to the α-galactomannan layer. The vegetative cell walls lack chitin, but chitin has been found as a component of the conidial cell wall. Melanin is not present in the Sz. pombe cell wall. The Sz. pombe cell wall proteins have both N-, and O-linked galactomannans [11].
The cell wall of Kluyveromyces lactis is similar to this of S. cerevisiae with the outer layer formed of mannoproteins and the inner of glucan [15]. β-1,3-glucan builds up to 50% of cell wall dry weight, while chitin contributes with only 1–3% [16]. Contrary to S. cerevisiae, whose cell wall density and polysaccharide composition changed considerably when grown at different temperatures, pH, and on different carbon sources [17], K. lactis glucan content was according to the authors’ conclusion, not dependent on the carbon source [15]. S. cerevisiae cell walls were considerably thicker than those of K. lactis when cells were grown on glucose. When K. lactis was grown on ethanol, cell wall showed similar thickness to that of S. cerevisiae but had a considerably increased sensitivity to Zymolyase speaking in favor of increased β-1,3-glucan concentration. The thickness of the S. cerevisiae cell wall does not increase upon growth on ethanol, but it becomes slightly less sensitive to the Zymolyase. Furthermore, S. cerevisiae cell wall mannose content increases when cells were grown on ethanol, while the mannose content of K. lactis cell wall decreased under the same conditions [15]. Since Zymolyase sensitivity is generally connected with the amount of β-1,3-glucan, walls containing more β-1,3-glucan being more sensitive, results indicate that different yeasts respond differently to environmental changes in terms of cell wall components’ regulation. In this way, they are able to adapt their walls to different environmental influences according to yeasts’ lifestyles.
2.2. Enzymatic Activities Involved in the β-1,3-Glucan Synthesis
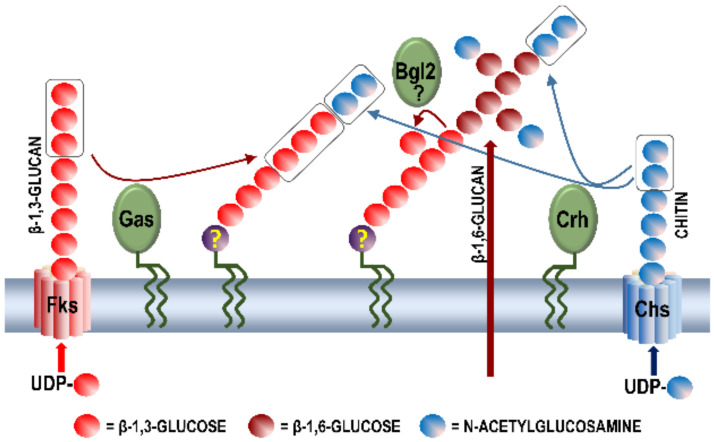
β-1,3-Glucan is the main component of all characterized fungal cell walls that is responsible for the osmotic stability of the cell. It is a branched polymer with branching in the β-1,6-positions making between 30% and 80% of the cell wall mass [5,18]. The β-1,3-glucan helix acts as a coiled spring-like structure giving elasticity and tensile strength to the cell wall [4]. It is synthesized by β-1,3-glucan synthases localized in the plasma membrane, which extrude newly synthesized linear glucan into the cell wall. The enzyme adds glucose residues using cytoplasmic UDP-glucose as the substrate, to the non-reducing end of the growing linear glucan polymer. The polymer is secreted through a channel formed by the enzyme transmembrane domains into the cell wall. C. neoformans and C. albicans have a single β-1,3-glucan synthase, Fks1, while S. cerevisiae has three genes coding for glucan synthases, but Fks1 synthetizes most of β-1,3-glucan during yeast vegetative growth [5]. Sz. pombe has four genes coding for β-1,3-glucan synthases, but only one of them has been shown to be involved in septation, mating, polarized growth, and spore formation and germination [19]. Morphology and growth rate of fks1 mutants are dramatically affected [5]. Fks1 in S. cerevisiae co-localizes to areas of polarized growth together with actin patches [20], while in Sz. pombe it localizes to the septum [19]. Rho1 protein, a part of the MAP kinase signaling pathway that regulates growth and the cell wall integrity response, acts as a regulatory subunit of Fks1 activating the synthesis of glucan when cells are growing, or during the cell wall stress, and inactivating it when it is not needed [4]. This model of β-1,3-glucan synthesis implies that glucan synthases use an intracellular source of UDP-glucose without the need for UDP-glucose transport across the membrane. Glycosidic linkages are formed within the space created by transmembrane domains of the enzyme by adding glucose units to the reducing end of the chain, while the ready polysaccharide protrudes through the synthase into the surrounding space forming the inner layer of the wall (Figure 1).
Figure 1.
Synthesis of carbohydrate components of the Saccharomyces cerevisiae cell walls. Glucan and chitin are synthesized by plasma membrane-associated β-1,3-glucan synthases Fks1-3 and chitin synthases Chs1-3, respectively. Both polymers are simultaneously excreted into the cell wall. Cell wall glycosylphosphatidylinositol (GPI)-anchored enzymes of the Gas and Crh families rearrange wall polysaccharides by transferring parts of protruding β-1,3-glucan and chitin, respectively, to existing β-1,3-, or β-1,6-glucans in the wall. Cell wall non-covalently bound enzyme Bgl2 has a role in branching of β-1,3-glucan chains.
Since the β-1,3-glucan molecules consist of very long chains of up to 1500 glucose units, it is difficult to envisage that such long chains could be made without eventually blocking the enzymatic activity of glucan synthase. Indeed, there is evidence that proteins of the Gas family possess the transglucosidase activity and that they transfer portions of the β-1,3-glucan protruding from the glucan synthase to the already synthesized glucan chain, liberating in this way the enzyme for further elongation of the glucan chain (Figure 1). How, or if at all, β-1,3-glucan chains are anchored to the cell membrane is still not known. Speculations that the putative anchor moiety involves a glycolipid or a membrane protein have never been experimentally corroborated. Another opened question is whether β-1,3-glucan chains are somehow covalently cross-linked to form a network similar to the bacterial peptidoglycan. A putative cross-linker should be a bivalent molecule able to connect two glucan chains, thus it could not be a carbohydrate. It has been proposed that some proteins that are covalently attached to glucan can form more than one link, bridging different carbohydrate chains [21]. However, knockouts of corresponding genes did not bring about the expected phenotype. Thus, this question still remains open.
2.3. Enzymatic Activities Involved in the β-1,6-Glucan Synthesis
β-1,6-Glucan is a significant element of cell walls of many yeasts including C. albicans and S. cerevisiae [4,5,22]. In S. cerevisiae, it is cross-linked with β-1,3-glucan, chitin, and with the GPI anchored proteins [23,24], playing an important role that is still not completely understood but seems to include interconnecting different wall components [4,5]. However, the β-1,6-glucan synthase has not yet been definitively identified in any fungal species. In S. cerevisiae a number of proteins that play roles in the synthesis of β-1,6-glucan have been identified [4], including Kre1, Kre5, Kre6, Kre9, Knh1, Big1, Rot1, and Skn1. Kre5, Big1, and Rot1 proteins are localized in the ER and probably participate in the control of the transport of proteins between ER and Golgi through protein-folding/quality control mechanisms. On the other hand, Kre6 and Skn1 are structurally similar to glycosylhydrolases/transglycosidases and according to that might have a role in cross-linking the β-1,6-glucan with other cell wall components. However, Kre6 and Skn1 are localized in the Golgi, and it is hard to expect that they have a role in cell wall matrix formation. Kre1 is GPI-bonded cell wall protein. The β-1,6-glucan of the kre1 mutant cell wall is shorter, suggesting that Kre1 functions in the cell wall to elongate β-1,6-glucans [25]. Kre9 and Knh1 are cell wall proteins that have a function in crosslinking β-1,6-glucan into the wall [4]. Simultaneous deletion of KNH1 and KRE9 is lethal. It is assumed that these enzymes have a role in the cross-linking of cell wall components or in the folding and targeting of the putative β-1,6-synthase to the plasma membrane. It has been shown that the β-1,6-glucan is synthesized at the plasma membrane [26], thus it is expected that the β-1,6-synthase will have a mechanism similar to the β-1,3-glucan and chitin synthases. β-1,6-Glucans have not been found in the cell wall of the fungi Aspergillus fumigatus [27] and Neurospora crassa [28], indicating that the role of β-1,6-glucan in the cell wall of different fungal species is not universal.
2.4. Enzymatic Activities Involved in Chitin Synthesis
Yeast species generally contain 1–2% of the cell wall mass made of chitin, while filamentous fungi may have up to 15% of chitin in their cell walls. Plasma membrane-associated chitin synthases use cytosolic UDP-N-acetylglucosamine as a substrate to synthesize linear polymers of β-1,4-N-acetylglucosamine by the addition of N-acetylglucosamine to the non-reducing end. Chitin is simultaneously excreted into the cell wall through multiple transmembrane domains of the chitin synthases forming a channel, reducing end first (Figure 1). In the cell wall, chitin chains are bonded by hydrogen bonding and assemble into microfibrils [7]. Extruded chitin can be deacetylated by chitin deacetylases to generate chitosan. Chitin polymer significantly contributes to the cell wall integrity, and disturbed chitin synthesis results in deformed and osmotically unstable yeast cells. All characterized fungi have chitin synthases and most have multiple genes encoding chitin synthases. Most frequently, one or two of the chitin synthases synthesize the majority of chitin, while others produce only smaller quantities [29,30]. S. cerevisiae has three chitin synthases: Chs1, Chs2, and Chs3 [31]. The Chs3 chitin synthase generates 80–90% of chitin [32] in the wall including chitin covalently cross-linked to β-1,3-glucan and the chitin ring formed during bud formation [24]. The chs3 mutant has defects in cell wall integrity and highly reduced levels of chitin, as well as very slow growth. Chs1 replenish chitin lost during cytokinesis, and Chs2 has a role in the formation of the primary septum. The simultaneous deletion of CHS1, CHS2, and CHS3 results in a lethal phenotype [33]. C. albicans has four [34] and Sz. pombe two chitin synthase genes [35], one of which lacks synthase activity [36]. Deletions of the major chitin synthases in C. albicans result in a weakened cell wall and reduced growth rate. In contrast, deletion of chs1 and chs2 in Sz. pombe does not affect growth rate and chitin was not detected in the Sz. pombe vegetative cell wall [37]. However, the Chs1 is required for the asci formation, while the Chs2 is involved in the septum formation [35,38]. Generally, biosynthesis of β-1,3-glucan and chitin share the same cell strategy and it can be assumed that the corresponding enzymatic mechanisms are used for the synthesis of other fungal wall polysaccharides, as well.
3. Yeast Cell Wall Proteomes
The development of bioinformatics and genome sequencing enabled much faster and easier identification and characterization of cell wall proteins. A number of potential GPI-anchored cell wall proteins were identified by bioinformatic methods [39,40], and by mass spectrometry [3,37,41]. Mass spectrometry was applied on the cell wall proteins isolated by chitinase and glucanase and digested by trypsin, or on the proteins isolated by “trypsin shaving” in which cell walls were trypsinized without previous isolation of the proteins and the released tryptic fragments were subjected to mass spectrometric analysis. Lately, glycosidic bonds of glycoproteins were digested by trifluoromethanesulfonic acid to isolate the proteins from the cell wall, followed by trypsinization and nano-LC/MS/MS analysis of peptides obtained in this way [42]. The trifluoromethanesulfonic acid treatment allows to identify positions of N-glycosylation since the first glucosamine bonded to the asparagine is not released by this treatment, and tryptic peptides can be identified with the attached glucosamine. The O-glycosylation sites can be detected by NH4OH treatment of the cell wall proteins that removes the oligosaccharide and tags the attachment site with -NH3 groups [43]. Tagged proteins are then trypsinized and analyzed by mass spectrometry to identify the peptides containing the -NH3 tag. S. cerevisiae cell wall proteins belonging to glycosylhydrolase families 16, 17, and 72, proteins of the Pir (protein with internal repeat) family, and adhesins/flocculins have homologs in almost all sequenced fungal genomes. Such results indicate that they have general roles indispensable for yeast cells and have thus been evolutionary conserved. Proteins detected in the cell walls of different yeasts by proteomic investigations are summarized in Table 1.
Table 1.
Proteins isolated identified in cell walls of different yeasts.
| Saccharomyces cerevisiae | Candida albicans | Schizosaccharomyces pombe | Kluyveromyces lactis | Yarrowia lipolytica | |
|---|---|---|---|---|---|
| Cell wall proteins with enzyme activities | |||||
| Glucan transferases | Gas1, Gas2, Gas3, Gas4, Gas5 | Pga4, Phr1, Phr2 | Gas1, Gas4, Gas5 | KlGas1, KlGas3, KlGas5 | - |
| Glucanase homologs | Bgl2, Scw4, Scw10 | Bgl21, Scw1, Scw4, Mp65 | - | KlScw4 | - |
| Chitin transferases | Crh1, Crh2 | Crh11, Crh12, Utr2 | - | KlCrh1, KlUtr2 | YlCrh1, YlCrh2 |
| Chitinases | Cts1, Cts2, Cts3 | Cht1, Cht2, Cht3, Cht4 | - | KlCts1p | - |
| Mannanase homologs | Dfg5, Dcw1 | Dfg5, Dcw1 | - | - | - |
| Adhesins | |||||
| Cellular interactions | Aga1, Aga2, Sag1, Flo1, Flo5, Flo9, Flo10, Flo11 | Als1, Als2, Als3, Als5, Hwp1 | Map4 | KlMuc1a, KlMuc1b, KlFlo5 | - |
| Proteins with unidentified functions | |||||
| Proteins linked to glucan through Pir-sequences | Pir1, Pir2, Pir3, Pir4 | Pir1 | - | KlPir1a, KlPir1b | Ylpir1 |
| GPI-anchored proteins | Ecm33, Ccw12, Ccw14, Sed1, Tir1-Tir4, Tip1, Cwp1, Cwp2, Dan1-Dan4, Fit1-Fit3, Spi1, Yps1-3, Yps6, Yps7 | Ecm33, Pga24, Pga29, Pga30, Pga45,÷Ssr1/Ccw14, Rbt1 | Ecm33, Meu10, Pwp1 | KlEcm33, KlCcw14, KlCwp1a, KlCwp1b, KLLA0E24959g, KLLA0E24893g, KLLA0B14498g, KlYps1, KlYps7 KLLA0D01507g | Ylcwp1 |
| Proteins with unknown linkage | Psu1, Als1 | Ywp1 | |||
Bioinformatic comparison of genes coding for 187 proteins involved in the biosynthesis of cell walls of different yeast strains/genera indicated that the variability of cell wall proteomes correlated with the taxonomic distance of the compared proteomes [44,45]. As cell walls of all yeasts share the same structural principles, it can be assumed that the proteins involved in the biosynthesis and remodeling of wall polysaccharides were rather conserved throughout the evolution. On the other hand, cell walls are also involved in the communication of the cell with its surrounding. For this purpose, yeasts need a more variable set of proteins that would reflect different environmental conditions and lifestyles.
3.1. Cell Wall Proteins with Enzyme Activities
A major part of the so far known cell wall proteins is thought to have a role in attachments and rearrangements of various elements of the wall. Some of them function as glycosylhydrolases and others as transglycosidases that synthesize new glycosidic bonds between the cleaved polymers [46,47]. Genes coding for these enzymes are conserved in fungal genomes. In most cases, these enzymes are encoded by multiple genes organized in families providing the redundancy of cross-linking activity and ensuring the functionality of the cell wall even if one of the genes is functionally lost by mutation. Indeed, genetic analyses show that in most cases the loss of a single enzyme does not result in a significant change in phenotype. Sometimes, the result is a decreased growth rate or increased sensitivity to cell wall perturbation agents. More apparent phenotype usually occurs in multiple mutants in which more or all members of the gene/protein family are deleted.
Enzymes of the Gas/Phr family are among the best characterized cell wall enzymes. These GPI-anchored enzymes are capable of rearranging β-1,3-glucan [47] and generating non-reducing ends in β-1,3-glucan for attachment of β-1,6-glucosylated mannoproteins [48]. S. cerevisiae has five GAS genes [49], C. albicans five homologous PHR genes [50], K. lactis cell wall proteome comprises three homologs of S. cerevisiae Gas proteins, KlGas1, KlGas3, and KlGas5 [15], and Sz. pombe has four GAS genes [51] having overlapping specificity. In S. cerevisiae, Gas1 and Gas5 are expressed in vegetative cells, while Gas2 and Gas4 are sporulation-specific [52]. Deletion of GAS1 results in enlarged spherical cells, increased quantity of β-1,3-glucan secreted to the medium, and reduced level of glucan in the cell wall [53]. Single GAS2 or GAS4 deletion has no effect on sporulation, but gas2gas4 double deletion results in the severely disturbed formation of the spore cell wall, indicating that their activities are redundant [49,54]. In C. albicans, Phrl and Phr2 are required for normal morphology and virulence and their expression is pH-dependent, with Phr2 being expressed at acidic and Phr1 at neutral to alkaline pH [48]. Phr1 is required for the adhesion to epithelial cells and abiotic substrates [50]. According to that the consequence of deletion of the encoding genes is pH-conditional defects in cell morphology and virulence [48]. In Sz. pombe, Gas4 is essential for maturation of ascospore wall, and Gas1 and Gas5 are most probably involved in elongation of β-1,3-glucan chains [51].
The Crh/Utr family has been identified as one of the major cell wall proteins family in the cell walls of S. cerevisiae and C. albicans. This family of transglycosidases has a role in the addition of short chitin chains to the non-reducing ends of β-1,6-glucan [46], and β-1,3-glucan [55] in S. cerevisiae and C. albicans. S. cerevisiae has two homologs, Crh1 and Crh2 [46] and C. albicans has three enzymes, Crh11, Crh12, and Utr2 [55]. Crh11 and Crh12 are the homologs of S. cerevisiae proteins Crh1 and Crh2 [46,56]. Utr2 seems to be a β-glucanase included in filamentation, adherence, and virulence [57]. K. lactis cell wall proteome contains Crh1 (KlChr1) and Utr2 (KlUtr2) as well [15]. Utr2 is found in K. lactis cell wall exclusively in logarithmic growth phase on either glucose or lactose or ethanol. In the cell wall of Y. lipolytica, Hwang et al. [58] found two cell wall proteins YlCrh1 and YlCrh2 showing high amino acid sequence similarity to S. cerevisiae Crh1 and Crh2. Deletion of the YlCRH1 and YlCRH2 resulted in increased sensitivity to Congo red and Calcofluor white. The YlCrh1 and YlCrh2 showed to be able to degrade β-1,3 glycosidic linkage. The YlCrh1 protein showed to be capable to hydrolyze β-1,4- and β-1,6-linkages as well, although with lower specific activities than that on β-1,3-linkage. Both YlCrh1 and YlCrh2 cannot degrade the substrate of exo-β-1,3-glucanases (4-nitrophenyl-β-D-glucopyranoside), indicating that they are endo β-1,3-glucanases [58].
Members of Bgl2/Scw4/Scw10/Scw11 family of enzymes homologous to plant β-1,3-glucanases have been found in S. cerevisiae and C. albicans cell walls. S. cerevisiae and C. albicans Bgl2 cleave glucose units from the reducing end of the β-(1,3)-oligosaccharide, transferring enzyme-bound oligosaccharide to an acceptor β-(1,3)-oligosaccharide, either at C-6 of the non-reducing end or at C-6 of an internal glucose unit [59]. The S. cerevisiae bgl2 mutant has higher level of chitin in the wall [60]. Deletion of bgl2 in C. albicans results in a disturbed cell wall and lower virulence [61]. In contrast to C. albicans and S. cerevisiae, Bgl2 homologs were not found in the Sz. pombe cell wall. S. cerevisiae single scw4 and scw10 mutants are sensitive to cell wall destabilizing agents like Calcofluor white and Congo red, and the scw4scw10 mutant shows a synergistic effect [62]. Grbavac et al. [63] demonstrated that Scw4 undergo double processing, by yapsins and Kex2 protease. Processing at the yapsin site significantly lowers the potential for covalent attachment of Scw4 to glucan. Furthermore, the overproduction of a fully processed form of Scw4 leads to high mortality, particularly in the stationary phase of growth, and to markedly increased cell size. The physiological role of Scw4 and Scw10 are still unknown. Two additional members of this family, Scw1 and Mp65, were detected in the cell walls of C. albicans. Castillo et al. [64] detected Scw1 protein in the cell wall of C. albicans that lacks a GPI motif, and it possesses glucanase activity. It has been proposed that the formation of disulfide bridges might be a mechanism of the binding Scw1 to the cell wall [64]. K. lactis cell wall proteome contains Scw4 (KlScw4) protein as well [15].
S. cerevisiae cell wall proteins Dfg5 and Dcw1 are homologs of N. crassa α-1,6-mannanases Dfg5 and Dcw1. Maddi and Free [28] established that in the N. crassa cell wall the α-1,6-mannose core of N-linked galactomannan was required for the covalent binding of cell wall proteins. Dfg5 and Dcw1 in this fungi cleave the N-linked α-1,6-mannan of mannoproteins and create new glycosidic bonds with cell wall glucans, so the dfg5dcw1 double mutant is unable to incorporate cell wall proteins [65]. According to that, it has been speculated that the functions of Dcw1 and Dfg5 in S. cerevisiae cell wall might also be related to the transfer of GPI-proteins from the membrane to the cell wall [66]. However, Dfg5 and Dcw1 in S. cerevisiae are shown to be crucial for the formation of the cell wall in growing buds [67], but the exact enzymatic functions of Dcw1p and Dfg5p are still unknown. Dfg5 mutants of C. albicans are shown to be defective in hypha formation [68]. Dfg5 and Dcw1 have overlapping functions in S. cerevisiae and C. albicans, as well as in N. crassa [65,66,67,68].
S. cerevisiae has three and C. albicans four chitinase genes [69,70]. In S. cerevisiae, Cts1 functions in degrading chitin synthesized between the mother and daughter cells as the primary septum [71], so in cts1 mutant, the division of mother and daughter cells is affected. In C. albicans, deletion of Cht3 results in a similar cell separation phenotype [70], while endochitinase 2, Cht2, might have a role in cell separation after cytokinesis since it was shown to be located close to the wall that separates daughter from mother cell [61]. Colussi et al. [72] characterized K. lactis chitinase KlCts1p. They showed that disruption of CTS1 resulted in a separation defect phenotype and that KlCts1p could restore normal morphogenesis to S. cerevisiae cts1 mutant cells, indicating identical roles of these two proteins.
Generally, this short overview clearly points out the complexity and variability of enzymatic activities present in the yeast cell wall. Enzymes that are capable of splitting and creating new glycosidic bonds are quite numerous, have different specificities, and are fairly conserved among different yeasts. Notably, for some of them, activities have only been demonstrated in vitro, or not at all, so that their exact physiological function still remains unclear. Yet, it can be assumed that they contribute to the pronounced plasticity and flexibility of the yeast cell wall.
3.2. Adhesins
Adhesins are crucial for biofilm formation and cell adhesion, allowing pathogenic fungi to start the infection process, or saprophytic fungi to stick to their nutrient-rich substrates. A number of adhesins have been identified in fungal cell walls. S. cerevisiae produces a- and α-agglutinin during mating that enables cell–cell interactions [73]. The a-agglutinin is expressed by mating-type cells in response to mating pheromone and consists of two subunits, Aga1 and Aga2. The carboxyl terminus of Aga2 has a high affinity for binding of α-agglutinin. The α-agglutinin, Sag1, is expressed in mating-type α cells and binds to Aga2 through its N-terminal region. S. cerevisiae also has five FLO genes four of which are coding for lectin-like proteins (Flo1, Flo5, Flo9, Flo10) that bind to mannose and/or glucose [74] enabling cell adhesion and flocculation. Flo11 on the other hand has a role in biofilm formation and recognizes a variety of other substrates [75] like agar and plastic surfaces, probably facilitating adhesion to plant materials in nature. K. lactis cell wall proteome contains a homolog of Flo5 (KLLAOE14586g) and two isoforms of Muc1/Flo11, while S. cerevisiae contains only one form of that protein [15]. KlFlo5 is preferentially found during growth in the presence of lactose. KlMuc1a predominates in the logarithmic phase and KlMuc1b in the stationary phase. Furthermore, KlMuc1a is more represented upon growth on glucose, while KlMuc1b seems to be lactose-induced.
In Sz. pombe, the Map4 protein facilitates cell–cell interactions during mating, and no other adhesins were found [76]. C. albicans cell wall contains different types of proteins such as adhesins and lectins, that allow attachment of the cells to different surfaces, and different enzymes involved in the degradation of the protective structures of host tissues or involved in the synthesis of the cell wall itself. Some of the proteins are bound in the cell wall non-covalently, whereas others are covalently bound to other cell wall proteins or to different cell wall polysaccharides [5,7]. Cell adhesion has been widely investigated in C. albicans as a human pathogen [74]. It was found that C. albicans genome contained a family of eight ALS genes coding for adhesins [77], among which Als1 and Als5 were demonstrated to bind a broad range of peptides [78], while Als3 had a crucial role in the biofilm formation and can also facilitate binding to host tissues [79] but is not required for virulence [80]. Als1 and Als10/Als2 were shown to be involved in epithelial adhesion and invasiveness due to their adhesion properties [81]. C. albicans Hwp1 adhesin enables adhesion to human skin and oral epithelia and has a role in the formation of biofilms [82].
3.3. Cell Wall Proteins with still Unidentified Functions
Cell wall proteins without any apparent enzyme activities have been identified in a number of yeast species suggesting that they might have non-enzymatic, or structural functions. It has to be noted, however, that the designation of these proteins as “structural” merely relates to the fact that no other function has been attributed to them so far. This group of proteins shows lower levels of homology across the yeast species and genera than the cell wall enzymes. Most of them are attached to the cell wall glucan through the GPI remnants. Since the GPI anchor plays a vital role in the transport of GPI-anchored proteins through the secretory pathway, mutations in genes encoding the proteins required for the biosynthesis of the GPI anchor are lethal in S. cerevisiae [40,83] and in Sz. pombe [84], while in C. albicans biosynthesis of GPI anchor is vital for normal morphology and virulence [85].
The structural cell wall protein most broadly represented among yeast genera is Ecm33. Mutants lacking Ecm33 induce the cell wall integrity (CWI) pathway and show altered cell wall architecture in both S. cerevisiae [86], Sz. pombe [87] and C. albicans [88]. In S. cerevisiae deletion of ECM33 results in spherical and swollen cells, highly sensitive to Calcofluor white and Congo red [86,89]. Ecm33 is found to be essential for normal cell wall architecture and integrity, and for the function and expression of some cell surface proteins in C. albicans [88,89]. ecm33 mutants of C. albicans have swollen and spherical cells highly sensitive to cell wall-perturbing agents [86,89]. There are two ECM33 paralogues in the genome of Sz. pombe (Meu10 and Ecm33). Ecm33 has yet unidentified but critical role in keeping the integrity of Sz. pombe cell wall [87].
Furthermore, S. cerevisiae cell wall GPI proteins comprise members of the TIR family (Tir1-Tir4, Tip1, Cwp1, Cwp2, and Dan1-Dan4) [90], FIT family (Fit1-Fit3) [91], yapsins family [92,93], and proteins Spi1, Sed1, Ccw12, and Ccw14. The physiological functions of these proteins are still mostly unknown. Cwp1 and Cwp2 are repressed while the other members of the TIR family are induced under anaerobic conditions [94]. tir1, tir3, and tir4 mutants under anaerobic conditions show growth defects. Members of the FIT family possibly have a role in binding iron from the medium [91], while Spi1 and Sed1 were proposed to have a protective role under glucose limitation [95]. The roles of Ccw12 and Ccw14 are still unknown. It was shown that ccw12 mutant has increased mortality in the stationary phase of growth [96,97]. Yapsins are GPI-anchored family of five aspartyl proteases (Yps1-3, Yps6, Yps7) [92,93] with substrate specificity similar to the Kex2 protease. A mutant lacking all five yapsins has a considerably reduced quantity of β-1,3- and β-1,6-glucan in the cell wall and undergoes lysis at 37 °C [98].
Proteomic analysis of hyphal and yeast form cells of C. albicans shows that there are some protein differences between these two cell types [42,99,100]. Castillo et al. [64] found 21 cell wall surface proteins in C. albicans proteome containing a cell wall signal in their immature form. Proteins Als10, Bgl21, Pga30, Pga31, Pga45, Phr2, Rbt1, and Utr2 were identified in C. albicans cell wall for the first time in this investigation [64]. Out of the 21 identified proteins by Castillo et al. [64], only Bgl21 and Scw1 were found not to contain a GPI-binding domain, while all the others do. Proteins Als1, Pga24, Pga30, Pga45, Rbt1, and superoxide dismutase Pga2 were identified with a single peptide, indicating that these proteins are present in the cell wall in smaller quantities. Pga2, Pga4, Pga24, Pga29, Pga30, Pga31, and Pga45 are predicted GPI proteins of the same family [39,101]. Pga2 is a superoxide dismutase, homologous to S. cerevisiae Yjr104c, with a function in ROS removal. Pga24 and Pga29 are proteins with unidentified roles. Pga30 is similar to the S. cerevisiae Sed1, and Pga45 shows a low similarity to a glucan 1,4-α-glucosidase Yil169c. Pga31 is assumed to be a membrane protein with low similarity to glucan 1,4-α-glucosidase and exo-α-sialidase [102]. Ssr1 might be a structural protein, without catalytic function found so far [101].
In the cell wall of Sz. pombe, six covalently bound cell wall proteins were identified by tandem mass spectrometry, including two alkali-extractable (Psu1 and Asl1) and four GPI-bonded cell wall proteins (Gas1, Gas5, Ecm33, and Pwp1) [37]. Pwp1 is an abundant structural GPI-bonded protein with the still unknown role. Psu1 is a homolog of the S. cerevisiae Sun family, while Asl1 has no homologs in S. cerevisiae. Sz. pombe cells with deleted PSU1 are resistant to β-1,3-glucanase digestion and unable to complete cell division [103]. Although the overall quantity of proteins in the cell walls of Sz. pombe is slightly lower, the level of their glycosylation is significantly lower than that in C. albicans and S. cerevisiae.
The cell wall proteome of K. lactis shares several features with the wall proteomes of S. cerevisiae, C. albicans, and C. glabrata. They have a similar number of covalently linked proteins [97], and their wall proteome composition depends on the growth conditions. Furthermore, the number of GPI proteins in the wall is much bigger than the number of Pir proteins. K. lactis cell wall proteome contains some species-specific proteins with the yet unknown role (KLLA0E24959g, KLLA0E24893g and KLLA0B14498g) but comprises some homologs of those of S. cerevisiae including yapsins (KlYps1, KlYps7 and KLLA0D01507g-homolog of ScYps3), Ccw14 (KlCcw14), and Cwp1 (KlCwp1a and KlCwp1b) as well [15]. Putative cell wall proteins in K. lactis, such as KLLA0E24959g, KLLA0E24893g, and KLLA0B14498g, lack a counterpart in the yeast databases. Potential GPI cell wall protein KLLA0E24893g with the unidentified role and with no apparent homolog in S. cerevisiae is exclusively found upon growth on ethanol. KLLA0B14498g and KLLA0E24959g are found to be more represented in the walls upon growth on glucose. In contrast, KlCcw14 seems to be ethanol-specific [15]. Although S. cerevisiae usually displays a higher degree of redundancy in enzymatic functions than K. lactis [104], K. lactis contains two isoforms of Cwp1, while S. cerevisiae contains only one form of that protein. The GPI protein KlCwp1a possesses a Pir repeat as well as its homolog ScCwp1, while KlCwp1b does not, suggesting that KlCwp1a can bind through its GPI anchor to β-1,6-glucan and through its Pir repeat to β-1,3-glucan [105,106]. Both isoforms of Cwp1 in K. lactis are predominantly found in the stationary phase.
Yarrowia lipolytica grows as a combination of short mycelial and yeast-like cells and has a high capacity to metabolize lipids and hydrocarbons to secrete heterologous proteins and to accumulate large amounts of organic acids. This made it interesting for industrial application and for the production of heterologous proteins. However, relatively little is known about its cell wall structure, especially about its cell wall proteins. Ramon et al. [107] characterized Ywp1 protein to be covalently linked to the mycelial cell wall, although it does not contain specific Pir- or GPI- binding motifs. Ywp1 is neither O-glycosylated nor N-glycosylated [108]. According to the results obtained by Ramon et al. [107], one part of Ywp1 is bound covalently directly to the glucan network or by disulfide bridges to other cell wall proteins, and other part that is bound to β-mercaptoethanol-extractable N-glycosylated high molecular weight proteins. Deletion of YWP1 has no effect on the cell growth rate, morphology, or the general structure of the cell wall. Furthermore, Jaafar and Zueco [109] identified Ylcwp1 cell wall protein containing a putative signal peptide and GPI-attachment signal and some other structural features, suggesting that YlCWP1 encodes a GPI-cell wall protein of Y. lipolytica.
Pir protein family has been found in S. cerevisiae and C. albicans, as well as in many other budding yeasts, but not in Sz. pombe. Pir proteins possess Kex2 cleavage sites, multiple internal repeats, and a cysteine domain at their C-terminus [103]. The connection between the protein and β-1,3-glucan is in the form of an alkali sensitive glutamate–glucose ester linkage [105]. Mutants lacking single Pir proteins do not have severe cell wall defects. The quadruple S. cerevisiae pir1pir2pir3pir4 mutant has larger cells and shows increased susceptibility to cell wall perturbing reagents [21]. The Pir proteins have been shown to be responsible for S. cerevisiae resistance against a plant antifungal compound osmotin and are important for the survival of cells in the stationary growth phase [110,111]. Pir proteins comprise several potential binding sites for β-1,3-glucan [112], so they might have a role in crosslinking β-1,3-glucan chains and fortifying cell wall upon under stress [113]. This presumption is additionally corroborated by the finding that in C. albicans, which contains only one Pir protein, its role seems to be essential [114]. K. lactis cell wall proteome contains two Pir proteins (KlPir1a and KlPir1b) [15]. Jaafar et al. [115] characterized Y. lipolytica cell wall protein Ylpir1. Ylpir1 is the homolog of Pir4 cell wall protein of S. cerevisiae. Disruption of YlPIR1 resulted in slightly increased resistance to Zymolyase, Calcofluor white, and Congo red, but growth rate and cell morphology were not affected by this mutation [115].
4. Conclusions
The cell wall as the yeast’s outermost cellular structure defines cell shape, protects it from environmental stresses, and allows interactions of the cell with its environment. Some of these functions like the preservation of cell integrity and osmotic stability are universal and require concomitant activities of enzymes that have to be conserved in all fungi. Others are more species-specific and reflect different cell habitats and lifestyles. These characteristics require more specific cell wall enzymes/proteins, as well. In order to understand how exactly yeasts form their external armor and how this structure can withstand high osmotic pressures and still be flexible enough to allow growth, budding, and other cellular events, future investigations should reveal the physiological roles of cell wall proteins in more details. Besides, our comprehension of the variability of the cell wall composition and understanding of the molecular basis of cell wall integrity is crucial for the development of new biotechnological applications of fungi as well as the creation of new antifungal therapies. Examples of such applications are the use of antifungal drugs based on the inhibition of β-1,3-glucan synthesis [116,117], or expression of heterologous proteins directed to the cell wall and displayed at the cell surface [118], but further strategies applied in cell surface engineering could result in new biotechnology tools with various applications.
Author Contributions
Conceptualization, R.T. and M.L.; writing—original draft preparation, R.T.; writing—review and editing, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation grant IP-2019-04-2891.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schweigkofler W., Lopandic K., Molnár O., Prillinger H. Analysis of phylogenetic relationships among Ascomycota with yeast phases using ribosomal DNA sequences and cell wall sugars. Org. Divers. Evol. 2002;2:1–17. doi: 10.1078/1439-6092-00029. [DOI] [Google Scholar]
- 2.Giaver G., Chu A.M., Ni L., Connelly C., Riles L., Véronneau S., Dow S., Lucau-Danila A., Anderson K., Andre B., et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 3.Yin Q.Y., De Groot P.W.J., Dekker H.L., De Jong L., Klis F.M., De Koster C.G. Comprehensive proteomic analysis of Saccharomyces cerevisiae cell walls. J. Biol. Chem. 2005;280:20894–20901. doi: 10.1074/jbc.M500334200. [DOI] [PubMed] [Google Scholar]
- 4.Lesage G., Bussey H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006;70:317–343. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klis F.M., Boorsma A., de Groot P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23:185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- 6.Kollar R., Reinhold B.B., Petrakova E., Yeh H.J.C., Ashwell G., Drgonova J., Kapteyn J.C., Klis F.M., Cabib E. Architecture of the yeast cell wall: β(1,6)-glucan interconnects mannoprotein, β(1,3)-glucan, and chitin. J. Biol. Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Herrera J., Elorza M.V., Valentin E., Sentandreleu R. Molecular organization of the cell wall of Canidida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006;6:14–29. doi: 10.1111/j.1567-1364.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- 8.Chaffin W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008;72:495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osumi M., Sato M., Ishijima S.A., Konomi M., Takagi T., Yaguchi H. Dynamics of cell wall formation in fission yeast, Schizosaccharomyces pombe. Fungal Genet. Biol. 1998;24:178–206. doi: 10.1006/fgbi.1998.1067. [DOI] [PubMed] [Google Scholar]
- 10.Grun C.H., Hochstenbach F., Humbel B.M., Verkleij A.J., Sietsma J.H., Klis F.M., Kamerling J.P., Vliegenthart J.F.G. The structure of cell wall α-glucan from fission yeast. Glycobiology. 2005;15:245–257. doi: 10.1093/glycob/cwi002. [DOI] [PubMed] [Google Scholar]
- 11.Magnelli P.E., Cipollo J.F., Robbins P.W. A glucanase-driven fractionation allows redefinition of Schizosaccharomyces pombe cell wall composition and structure: Assignment of diglucan. Anal. Biochem. 2005;336:202–212. doi: 10.1016/j.ab.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Hochstenbach F., Klis F.M., van den Ende H., van Donselaar E., Peters P.J., Klausner R.D. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. USA. 1998;95:9161–9166. doi: 10.1073/pnas.95.16.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katayama S., Hirata D., Arellano M., Perez P., Toda T. Fission yeast alpha-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J. Cell Biol. 1999;144:1173–1186. doi: 10.1083/jcb.144.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humbel B.M., Konomi M., Takagi T., Kamasawa N., Ishijima S.A., Osumi M. In situ localization of β-glucans in the cell wall of Schizosaccharomyces pombe. Yeast. 2001;18:433–444. doi: 10.1002/yea.694. [DOI] [PubMed] [Google Scholar]
- 15.Backhaus K., Heilmann C.J., Sorgo A.G., Purschke G., de Koster C.G., Klis F.M., Heinisch J.J. A systematic study of the cell wall composition of Kluyveromyces lactis. Yeast. 2010;27:647–660. doi: 10.1002/yea.1781. [DOI] [PubMed] [Google Scholar]
- 16.Kapteyn J.C., van den Ende H., Klis F.M. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta Gen. Subj. 1999;1426:373–383. doi: 10.1016/S0304-4165(98)00137-8. [DOI] [PubMed] [Google Scholar]
- 17.Aguilar-Uscanga B., Francois J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003;37:268–274. doi: 10.1046/j.1472-765X.2003.01394.x. [DOI] [PubMed] [Google Scholar]
- 18.Latge J.-P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 19.Cortes J.C.G., Ishiguro J., Duran A., Ribas C. Localization of the (1,3)β-D glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 2002;115:4081–4096. doi: 10.1242/jcs.00085. [DOI] [PubMed] [Google Scholar]
- 20.Utsugi T., Minemura M., Hirata A., Abe M., Watanabe D., Ohya Y. Movement of yeast 1,3-β-glucan synthase is essential for uniform cell wall synthesis. Genes Cells. 2002;7:1–9. doi: 10.1046/j.1356-9597.2001.00495.x. [DOI] [PubMed] [Google Scholar]
- 21.Mrsa V., Tanner W. Role of NaOH-extractable cell wall proteins Ccw5p, Ccw6p, Ccw7p and Ccw8p (members of the Pir protein family) in stability of the Saccharomyces cerevisiae cell wall. Yeast. 1999;15:813–820. doi: 10.1002/(SICI)1097-0061(199907)15:10A<813::AID-YEA421>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 22.Aimanianda V., Clavaud C., Simenel C., Fontaine T., Delepierre M., Latge J.-P. Cell wall β-(1,6)-glucan of Saccharomyces cerevisiae. J. Biol. Chem. 2009;284:13401–13412. doi: 10.1074/jbc.M807667200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapteyn J.C., Montijn R.C., Vink E., de la Cruz J., Llobell A., Douwes J.E., Shimoi H., Lipke P.N., Klis F.M. Retention of Saccharomyces cerevisiae cell wall proteins through a phosphodiester-linked β-1,3-/β-1,6-glucan heteropolymer. Glycobiology. 1996;6:337–345. doi: 10.1093/glycob/6.3.337. [DOI] [PubMed] [Google Scholar]
- 24.Kollar R., Petrakova E., Ashwell G., Robbins P.W., Cabib E. Architecture of the yeast cell wall—The linkage between chitin and beta(1,3)-glucan. J. Biol. Chem. 1995;270:1170–1178. doi: 10.1074/jbc.270.3.1170. [DOI] [PubMed] [Google Scholar]
- 25.Boone C., Sommer S.S., Hensel A., Bussey H. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J. Cell Biol. 1990;110:1833–1843. doi: 10.1083/jcb.110.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montijn R.C., Vink E., Muller W.H., Verkleij A.J., Van Den Ende H., Henrissat B., Klis F.M. Localization of synthesis of β-1,6-glucan in Saccharomyces cerevisiae. J. Bacteriol. 1999;181:7414–7420. doi: 10.1128/JB.181.24.7414-7420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gastebois A., Clavaud D., Aimanianda V., Latge´ J.-P. Aspergillus fumigatus: Cell wall polysaccharides, their biosynthesis and organization. Future Microbiol. 2009;4:583–595. doi: 10.2217/fmb.09.29. [DOI] [PubMed] [Google Scholar]
- 28.Maddi A., Free S.J. α-1,6-Mannosylation of N-linked oligosaccharide present on cell wall proteins is required for their incorporation into the cell wall in the filamentous fungus Neurospora crassa. Eukaryot. Cell. 2010;9:1766–1775. doi: 10.1128/EC.00134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mio T., Yabe T., Sudoh M., Satoh Y., Nakamina T., Arisawa M., Yamada-Okabe H. Role of three chitin synthase genes in the growth of Candida albicans. J. Bacteriol. 1996;178:2416–2419. doi: 10.1128/JB.178.8.2416-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munro C.A., Winter K., Buchan A., Henry K., Becker J.M., Brown A.J.P., Bulawa C.E., Gow N.A.R. Chs1 of Candida albicans is an essential chitin synthase required for synthesis of the septum and for cell integrity. Mol. Microbiol. 2001;39:1414–1426. doi: 10.1046/j.1365-2958.2001.02347.x. [DOI] [PubMed] [Google Scholar]
- 31.Roncero C. The genetic complexity of chitin synthesis in fungi. Curr. Genet. 2002;41:367–378. doi: 10.1007/s00294-002-0318-7. [DOI] [PubMed] [Google Scholar]
- 32.Valdivieso M.H., Mol P.C., Shaw J.A., Cabib E., Duran A. CAL1, a gene required for activity of chitin synthase 3 in Saccharomyces cerevisiae. J. Cell Biol. 1991;114:101–109. doi: 10.1083/jcb.114.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulawa C.E. Genetics and molecular biology of chitin synthesis in fungi. Ann. Rev. Microbiol. 1993;47:505–534. doi: 10.1146/annurev.mi.47.100193.002445. [DOI] [PubMed] [Google Scholar]
- 34.Munro C.A., Whitton R.K., Hughes H.B., Rella M., Selvaggini S., Gow N.A.R. CHS8—A fourth chitin synthase gene of Candida albicans contributes to the in vitro chitin synthase activity, but is dispensable for growth. Fungal Genet. Biol. 2003;40:146–158. doi: 10.1016/S1087-1845(03)00083-5. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo Y., Tanaka K., Nakagawa T., Matsuda H., Kawamukai M. Genetic analysis of chs1+ and chs2+ encoding chitin synthases from Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 2004;68:1489–1499. doi: 10.1271/bbb.68.1489. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Garcia R., Duran A., Valdivieso M.-H. In Schizosaccharomyces pombe chs2p has no chitin synthase activity but is related to septum formation. FEBS Lett. 2003;549:176–180. doi: 10.1016/S0014-5793(03)00812-3. [DOI] [PubMed] [Google Scholar]
- 37.De Groot P.W.J., Yin Q.Y., Weig M., Sosinska G.S., Klis F.M., De Koster C.G. Mass Spectrometric identification of covalently bound cell wall proteins from the fission yeast Schizosaccharomyces pombe. Yeast. 2007;24:267–278. doi: 10.1002/yea.1443. [DOI] [PubMed] [Google Scholar]
- 38.Arellano M., Cartagena-Lirola H., Hajibagheri M.A.N., Duran A., Valdivieso M.H. Proper ascospore maturation requires the chs1+ chitin synthase gene in Schizosaccharomyces pombe. Mol. Microbiol. 2000;35:79–89. doi: 10.1046/j.1365-2958.2000.01678.x. [DOI] [PubMed] [Google Scholar]
- 39.De Groot P.W.J., Hellingwerf K.J., Klis F.M. Genome-wide identification of fungal GPI proteins. Yeast. 2003;20:781–796. doi: 10.1002/yea.1007. [DOI] [PubMed] [Google Scholar]
- 40.Eisenhaber B., Maurer-Stroh S., Novatchkova M., Schneider G., Eisenhaber F. Enzymes and auxiliary factors for GPI lipid anchor biosynthesis and post-translational transfer to proteins. Bioessays. 2003;25:367–385. doi: 10.1002/bies.10254. [DOI] [PubMed] [Google Scholar]
- 41.Yin Q.Y., de Groot P.W.J., de Koster C.G., Klis F.M. Mass spectrometry based proteomics of fungal wall glycoproteins. Trends Microbiol. 2007;16:20–25. doi: 10.1016/j.tim.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Maddi A., Bowman S.M., Free S.J. Trifluoromethanesulfonic acid-based proteomic analysis of cell wall and secreted proteins of the ascomycetous fungi Neurospora crassa and Candida albicans. Fungal Genet. Biol. 2009;46:768–781. doi: 10.1016/j.fgb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rademaker G.J., Pergantis S.A., Blok-Tip L., Langridge J.I., Kleen A., Thomas-Oates J.E. Mass spectrometric determination of the sites of O-glycan attachment with low picomolar sensitivity. Anal. Biochem. 1998;257:149–160. doi: 10.1006/abio.1997.2548. [DOI] [PubMed] [Google Scholar]
- 44.Coronado J.E., Mneimneh S., Epstein S.L., Qiu W.G., Lipke P.N. Conserved processes and lineage-specific proteins in fungal cell wall evolution. Eukaryot. Cell. 2007;6:2269–2277. doi: 10.1128/EC.00044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen X.X., Opulente D.A., Kominek J., Zhou X., Steenwyk J.L., Buh K.V., Haase M.A.B., Wisecaver J.H., Wang M., Doering D.T., et al. Tempo and mode of genome evolution in the budding yeast subphylum. Cell. 2018;175:1533–1545. doi: 10.1016/j.cell.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabib E., Blanco N., Grau C., Rodriguez-Pena J.M., Arroyo J. Crh1p and Crh2p are required for the cross-linking of chitin to β(1,6)glucan in the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2007;63:921–935. doi: 10.1111/j.1365-2958.2006.05565.x. [DOI] [PubMed] [Google Scholar]
- 47.Mouyna I., Fontaine T., Vai M., Monod M., Fonzi W.A., Diaquin M., Popolo L., Hartland R.P., Latgé J.P. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J. Biol. Chem. 2000;275:14882–14889. doi: 10.1074/jbc.275.20.14882. [DOI] [PubMed] [Google Scholar]
- 48.Fonzi W.A. PHR1 and PHR2 of Candida albicans encode putative glycosidases required for proper cross-linking of β-1,3-glucan and β-1,6-glucan. J. Bacteriol. 1999;181:7070–7079. doi: 10.1128/JB.181.22.7070-7079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragni E., Fontaine T., Gissi C., Latge J.P., Popolo L. The Gas family of proteins of Saccharomyces cerevisiae: Characterization and evolutionary analysis. Yeast. 2007;24:297–308. doi: 10.1002/yea.1473. [DOI] [PubMed] [Google Scholar]
- 50.Calderon J., Zavrel M., Ragni E., Fonzi W.A., Rupp S., Popolo L. PHR1, a pH regulated gene of Candida albicans encoding a glucan-remodeling enzyme, is required for adhesion and invasion. Microbiology. 2010;156:2484–2494. doi: 10.1099/mic.0.038000-0. [DOI] [PubMed] [Google Scholar]
- 51.De Medina-Redondo M., Arnaiz-Pita Y., Fontaine T., Del Rey F., Latge J.P., De Aldana C.R. The β-1,3-glucanosyltransferase Gas4p is essential for ascospore wall maturation and spore viability in Schizosaccharomyces pombe. Mol. Microbiol. 2008;68:1283–1299. doi: 10.1111/j.1365-2958.2008.06233.x. [DOI] [PubMed] [Google Scholar]
- 52.Rolli E., Ragni E., De Medina-Redondo M., Arroyo J., De Aldana C.R., Popolo L. Expression, stability, and replacement of glucan-remodeling enzymes during developmental transitions in Saccharomyces cerevisiae. Mol. Biol. Cell. 2011;22:1585–1598. doi: 10.1091/mbc.e10-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ram A.F., Kapteyn J.C., Montijn R.C., Caro L.H., Douwes J.E., Baginsky W., Mazur P., van den Ende H., Klis F.M. Loss of the plasma membrane-bound protein Gas1p in Saccharomyces cerevisiae results in the release of beta1,3-glucan into the medium and induces a compensation mechanism to ensure cell wall integrity. J. Bacteriol. 1998;180:1418–1424. doi: 10.1128/JB.180.6.1418-1424.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ragni E., Coluccio A., Rolli E., Rodriguez-Pena J.M., Colasante G., Arroyo J., Neiman A.M., Popolo L. GAS2 and GAS4, a Pair of Developmentally Regulated Genes Required for Spore Wall Assembly in Saccharomyces cerevisiae. Eukaryot. Cell. 2007;6:302–316. doi: 10.1128/EC.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pardini G., de Groot P.W.J., Coste A.T., Karababa M., Klis F.M., de Koster C.G., Sanglard D. The CRH family coding for cell wall glycosylphosphatidylinositol proteins with a predicted transglycosidase domain affects cell wall organization and virulence of Candida albicans. J. Biol. Chem. 2006;281:40399–40411. doi: 10.1074/jbc.M606361200. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Pena J.M., Rodríguez C., Alvarez A., Nombela C., Arroyo J. Mechanisms for targeting of the Saccharomyces cerevisiae GPI-anchored cell wall protein Crh2p to polarised growth sites. J. Cell Sci. 2002;115:2549–2558. doi: 10.1242/jcs.115.12.2549. [DOI] [PubMed] [Google Scholar]
- 57.Alberti-Segui C., Morales A.J., Xing H., Kessler M.M., Willins D.A., Weinstock K.G., Cottarel G., Fechtel K., Rogers B. Identification of potential cell-surface proteins in Candida albicans and investigation of the role of a putative cell surface glycosidase in adhesion and virulence. Yeast. 2004;21:285–302. doi: 10.1002/yea.1061. [DOI] [PubMed] [Google Scholar]
- 58.Hwang J.-S., Seo D.-H., Kim J.-Y. Soluble forms of YlCrh1p and YlCrh2p, cell wall proteins of Yarrowia lipolytica, have β-1,3-glycosidase activity. Yeast. 2006;23:803–812. doi: 10.1002/yea.1395. [DOI] [PubMed] [Google Scholar]
- 59.Aimanianda V., Simenel C., Garnaud C., Clavaud C., Tada R., Barbin L., Mouyna I., Heddergott C., Popolo L., Ohya Y., et al. The Dual Activity Responsible for the Elongation and Branching of β-(1,3)-Glucan in the Fungal Cell Wall. mBio. 2017;8:e00619-17. doi: 10.1128/mBio.00619-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalebina T.S., Farkas V., Laurinavichiute D.K., Gorlovoy P.M., Fominov G.V., Bartek P., Kulaev I.S. Deletion of BGL2 results in an increased chitin level in the cell wall of Saccharomyces cerevisiae. Antonie Van Leeuwenhoek. 2003;84:179–184. doi: 10.1023/A:1026034123673. [DOI] [PubMed] [Google Scholar]
- 61.Sarthy A.V., McGonigal T., Coen M., Frost D.J., Meulbroek J.A., Goldman R.C. Phenotype in Candida albicans of a disruption of the BGL2 gene encoding a 1,3-beta-glucosyltransferase. Microbiology. 1997;143:367–376. doi: 10.1099/00221287-143-2-367. [DOI] [PubMed] [Google Scholar]
- 62.Sestak S., Hagen I., Tanner W., Strahl S. Scw10p, a cell wall glucanase/transglucosidase important for cell wall stability in Saccharomyces cerevisiae. Microbiology. 2004;150:3197–3208. doi: 10.1099/mic.0.27293-0. [DOI] [PubMed] [Google Scholar]
- 63.Grbavac A., Čanak I., Stuparević I., Teparić R., Mrša V. Proteolytic processing of the Saccharomyces cerevisiae cell wall protein Scw4 regulates its activity and influences its covalent binding to glucan. BBA-Mol. Cell Res. 2017;1864:507–515. doi: 10.1016/j.bbamcr.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Castillo L., Calvo E., Martínez A.I., Ruiz-Herrera J., Valentín E., Lopez J.A., Sentandreu R. A study of the Candida albicans cell wall proteome. Proteomics. 2008;8:3871–3881. doi: 10.1002/pmic.200800110. [DOI] [PubMed] [Google Scholar]
- 65.Maddi A., Fu C., Free S.J. The Neurospora crassa dfg5 and dcw1 genes encode α-1,6-mannanases that function in the incorporation of glycoproteins into the cell wall. PLoS ONE. 2012;7:e38872. doi: 10.1371/journal.pone.0038872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitagaki H., Wu H., Shimoi H., Ito K. Two homologous genes, DCW1 (YKL046c) and DFG5, are essential for cell growth and encode glycosylphophatidylinositol (GPI)-anchored membrane proteins required for cell wall biogenesis in Saccharomyces cerevisiae. Mol. Microbiol. 2002;46:1011–1022. doi: 10.1046/j.1365-2958.2002.03244.x. [DOI] [PubMed] [Google Scholar]
- 67.Kitagaki H., Ito K., Shimoi H. A temperature-sensitive dcw1 mutant of Saccharomyces cerevisiae is cell cycle arrested with small buds which have aberrant cell walls. Eukaryot. Cell. 2004;3:1297–1306. doi: 10.1128/EC.3.5.1297-1306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spreghini E., Davis D.A., Subaran R., Kim M., Mitchell A.P. Roles of Candida albicans Dfg5p and Dcw1p cell surface proteins in growth and hypha formation. Eukaryot. Cell. 2003;2:746–755. doi: 10.1128/EC.2.4.746-755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adams D.J. Fungal cell wall chitinases and glucanases. Microbiology. 2004;150:2029–2035. doi: 10.1099/mic.0.26980-0. [DOI] [PubMed] [Google Scholar]
- 70.Dunkler A., Walther A., Specht C.A., Wendland J. Candida albicans CHT3 encodes the functional homolog of the Cts1 chitinase of Saccharomyces cerevisiae. Fungal Genet. Biol. 2005;42:935–947. doi: 10.1016/j.fgb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Kuranda M.J., Robbins P.W. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 1991;266:19758–19767. [PubMed] [Google Scholar]
- 72.Colussi P.A., Specht C.A., Taron C.H. Characterization of a Nucleus-Encoded Chitinase from the Yeast Kluyveromyces lactis. Appl. Environ. Microbiol. 2005;71:2862–2869. doi: 10.1128/AEM.71.6.2862-2869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang G., Dougherty S.D., Erdman S.E. Conserved WCPL and CX4C domains mediate several mating adhesion interactions in Saccharomyces cerevisiae. Genetics. 2009;182:173–189. doi: 10.1534/genetics.108.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dranginis A.M., Rauceo J.M., Coronado J.E., Lipke P.N. A biochemical guide to yeast adhesins: Glycoproteins for social and antisocial occasions. Microbiol. Mol. Biol. Rev. 2007;71:282–294. doi: 10.1128/MMBR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goossens K.V.Y., Willaert R.G. The N-terminal domain of the Flo11 protein from Saccharomyces cerevisiae is an adhesion without mannose-binding activity. FEMS Yeast Res. 2012;12:78–87. doi: 10.1111/j.1567-1364.2011.00766.x. [DOI] [PubMed] [Google Scholar]
- 76.Sharifmoghadam M.R., Valdivieso M.H. The Schizosaccharomyces pombe Map4 adhesin is a glycoprotein that can be extracted from the cell wall with alkali but not with beta-glucanases and requires the C-terminal DIPSY domain for function. Mol. Microbiol. 2008;69:1476–1490. doi: 10.1111/j.1365-2958.2008.06375.x. [DOI] [PubMed] [Google Scholar]
- 77.Verstrepen K.J., Klis F.M. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 2006;60:5–15. doi: 10.1111/j.1365-2958.2006.05072.x. [DOI] [PubMed] [Google Scholar]
- 78.Nobbs A.H., Vickerman M.M., Jenkinson H.F. Heterologous expression of Candida albicans cell wall-associated adhesins in Saccharomyces cerevisiae reveals differential specificities in adherence and biofilm formation and in binding oral Streptococcus gordonii. Eukaryot. Cell. 2010;9:1622–1634. doi: 10.1128/EC.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao X., Daniels K.J., Oh S.-H., Green C.B., Yeater K.M., Soll D.R., Hoyer L.L. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology. 2006;152:2287–2299. doi: 10.1099/mic.0.28959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cleary I.A., Reinhard S.M., Miller C.L., Murdoch C., Thornhill M.H., Lazzell A.L., Monteagudo C., Thomas D.P., Saville S.P. Candida albicans adhesin Als3p is dispensible for virulence in the mouse model of disseminated candidiasis. Microbiology. 2011;157:1806–1815. doi: 10.1099/mic.0.046326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheppard D.C., Yeaman M.R., Welch W.H., Phan Q.T., Fu Y., Ibrahim A.S., Filler S.G., Zhang M., Waring A.J., Edwards J.E., Jr. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 2004;279:30480–30489. doi: 10.1074/jbc.M401929200. [DOI] [PubMed] [Google Scholar]
- 82.Nobile C.J., Nett J.E., Andes D.R., Mitchell A.P. Function of Candida albicans adhesin Hwp1p in biofilm formation. Eukaryot. Cell. 2006;5:1604–1610. doi: 10.1128/EC.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kinoshita T., Inoue N. Dissecting and manipulating the pathway for glycosylphosphatidylinositol-anchor biosynthesis. Curr. Opin. Chem. Biol. 2000;4:632–638. doi: 10.1016/S1367-5931(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 84.Colussi P.A., Orlean P. The essential Schizosaccharomyces pombe gpil1+ gene complements bakers’ yeast GPI anchoring mutant and is required for efficient cell separation. Yeast. 1997;13:139–150. doi: 10.1002/(SICI)1097-0061(199702)13:2<139::AID-YEA69>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 85.Victoria G.S., Kumar P., Komath S.S. The Candida albicans homologue of PIG-P, CaGpi19p: Gene dosage and role in growth and filamentation. Microbiology. 2010;156:3041–3051. doi: 10.1099/mic.0.039628-0. [DOI] [PubMed] [Google Scholar]
- 86.Pardo M., Monteoliva L., Vazquez P., Martınez M.R., Molero G., Nombela C., Gil C. PST1 and ECM33 encode two yeast cell surface GPI proteins important for cell wall integrity. Microbiology. 2004;150:4157–4170. doi: 10.1099/mic.0.26924-0. [DOI] [PubMed] [Google Scholar]
- 87.Takada H., Nishida A., Domae M., Kita A., Yamano Y., Uchida A., Ishiwata S., Fang Y., Zhou X., Masuko T., et al. The cell surface protein gene ecm33þ is a target of the two transcription factors Atf1 and Mbx1 and negatively regulates Pmk1 MAPK cell integrity signaling in fission yeast. Mol. Biol. Cell. 2010;21:674–685. doi: 10.1091/mbc.e09-09-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinez-Lopez R., Park H., Myers C.L., Gil C., Filler S.G. Candida albicans Ecm33p is important for normal cell wall architecture and interactions with host cells. Eukaryot. Cell. 2006;5:140–147. doi: 10.1128/EC.5.1.140-147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martinez-Lopez R., Monteoliva L., Diez-Orejas R., Nombela C., Gil C. The GPI-anchored protein CaEcm33p is required for cell wall integrity, morphogenesis and virulence in Candida albicans. Microbiology. 2004;150:3341–3354. doi: 10.1099/mic.0.27320-0. [DOI] [PubMed] [Google Scholar]
- 90.Van Der Vaart J.M., Caro L.H.P., Chapman J.W., Klis F.M., Verrips C.T. Identification of three mannoproteins in the cell wall of Saccharomyces cerevisiae. J. Bacteriol. 1995;177:3104–3110. doi: 10.1128/JB.177.11.3104-3110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Protchenko O., Ferea T., Rashford J., Tiedeman J., Brown P.O., Botstein D., Philpott C.C. Three cell wall mannoproteins facilitate the uptake of iron in Saccharomyces cerevisiae. J. Biol. Chem. 2001;276:49244–49250. doi: 10.1074/jbc.M109220200. [DOI] [PubMed] [Google Scholar]
- 92.Komano H., Fuller R.S. Shared functions in vivo of a glycosylphosphatidylinositol-linked aspartyl protease, Mkc7, and the proprotein processing protease Kex2 in yeast. Proc. Natl. Acad. Sci. USA. 1995;92:10752–10756. doi: 10.1073/pnas.92.23.10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komano H., Rockwell N.C., Wang G.T., Krafft G.A., Fuller R.S. Purification and characterization of the yeast glycosylphosphatidylinositol anchored, mono-basic specific aspartyl protease yapsin 2 (Mkc7) J. Biol. Chem. 1999;274:24431–24437. doi: 10.1074/jbc.274.34.24431. [DOI] [PubMed] [Google Scholar]
- 94.Abramova N., Sertil O., Mehta S., Lowry C.V. Reciprocal regulation of anaerobic and aerobic cell wall mannoprotein gene expression in Saccharomyces cerevisiae. J. Bacteriol. 2001;183:2881–2887. doi: 10.1128/JB.183.9.2881-2887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimoi H., Kitagaki H., Ohmori H., Iimura Y., Ito K. Sed1p is a major cell wall protein of Saccharomyces cerevisiae in the stationary phase and is involved in lytic enzyme resistance. J. Bacteriol. 1998;180:3381–3387. doi: 10.1128/JB.180.13.3381-3387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mrša V., Ecker M., Strahl-Bolsinger S., Nimtz M., Lehle L., Tanner W. Deletion of new covalently linked cell wall glycoproteins alters the electrophoretic mobility of phosphorylated wall components of Saccharomyces cerevisiae. J. Bacteriol. 1999;181:3076–3086. doi: 10.1128/JB.181.10.3076-3086.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klis F.M., Brul S., Piet W.J., de Groot W.J. Covalently linked wall proteins in ascomycetous fungi. Yeast. 2010;27:489–493. doi: 10.1002/yea.1747. [DOI] [PubMed] [Google Scholar]
- 98.Krysan D.J., Ting E.L., Abeijon C., Kroos L., Fuller R.S. Yapsins are a family of aspartyl proteases required for cell wall integrity in Saccharomyces cerevisiae. Eukaryot. Cell. 2005;4:1364–1374. doi: 10.1128/EC.4.8.1364-1374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sosinska G.J., de Koning L.J., de Groot P.W.J., Manders E.M.M., Dekker H.L., Hellingwerf K.J., de Koster C.G., Klis F.M. Mass spectrometric quantification of the adaptations in the wall proteome of Candida albicans in response to ambient pH. Microbiology. 2011;157:136–146. doi: 10.1099/mic.0.044206-0. [DOI] [PubMed] [Google Scholar]
- 100.Heilmann C.J., Sorgo A.G., Siliakus A.R., Dekker H.L., Brul S., de Koster C.G., de Koning L.J., Klis F.M. Hyphal induction in the human fungal pathogen Candida albicans reveals a characteristic wall protein profile. Microbiology. 2011;157:2297–2307. doi: 10.1099/mic.0.049395-0. [DOI] [PubMed] [Google Scholar]
- 101.Garcera A., Martinez A.I., Castillo L., Elorza M.V., Sentandreu R., Valentín E. Identification and study of a Candida albicans protein homologous to Saccharomyces cerevisiae Ssr1p, an internal cell-wall protein. Microbiology. 2003;149:2137–2145. doi: 10.1099/mic.0.26301-0. [DOI] [PubMed] [Google Scholar]
- 102.Castillo L., Martinez A.I., Garcera A., Garcia-Martinez J., Ruiz-Herrera J., Valentín E., Sentandreu R. Genomic response programs of Candida albicans following protoplasting and regeneration. Fungal Genet. Biol. 2006;43:124–134. doi: 10.1016/j.fgb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Omi K., Sonoda H., Nagata K., Sugita K. Cloning and characterization of psu1+, a new essential fission yeast gene involved in cell wall synthesis. Biochem. Biophys. Res. Commun. 1999;262:368–374. doi: 10.1006/bbrc.1999.1209. [DOI] [PubMed] [Google Scholar]
- 104.Gbelska Y., Krijger J.J., Breunig K.D. Evolution of gene families: The multidrug resistance transporter genes in five related yeast species. FEMS Yeast Res. 2006;6:345–355. doi: 10.1111/j.1567-1364.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 105.Ecker M., Deutzmann R., Lehle L., Mrsa V., Tanner W. Pir proteins of Saccharomyces cerevisiae are attached to β-1,3-glucan by a new protein-carbohydrate linkage. J. Biol. Chem. 2006;281:11523–11529. doi: 10.1074/jbc.M600314200. [DOI] [PubMed] [Google Scholar]
- 106.Kapteyn J.C., ter Riet B., Vink E., Blad S., De Nobel H., van den Ende H., Klis F.M. Low external pH induces HOG1 dependent changes in the organisation of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2001;39:469–479. doi: 10.1046/j.1365-2958.2001.02242.x. [DOI] [PubMed] [Google Scholar]
- 107.Ramon A.M., Gil R., Burgal M., Sentandreu R., Valentin E. A novel cell wall protein specific to the mycelial form of Yarrowia lipolytica. Yeast. 1996;12:1535–1548. doi: 10.1002/(SICI)1097-0061(199612)12:15<1535::AID-YEA59>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 108.Ramón A.M., Valentín E., Maicas S., Sentandreu R. Expression of YWP1, a gene that encodes a specific Yarrowia lipolytica mycelial cell wall protein, in Saccharomyces cerevisiae. Fungal Genet. Biol. 1997;22:77–83. doi: 10.1006/fgbi.1997.1000. [DOI] [PubMed] [Google Scholar]
- 109.Jaafar L., Zueco J. Characterization of a glycosylphosphatidylinositol bound cell-wall protein (GPI-CWP) in Yarrowia lipolytica. Microbiology. 2004;150:53–60. doi: 10.1099/mic.0.26430-0. [DOI] [PubMed] [Google Scholar]
- 110.Yun D.-J., Zhao Y., Pardo J.M., Narasimhan M.L., Damsz D., Lee H., Abad L.R., D’Urzo M.P., Hasegawa P.M., Bressan R.A. Stress proteins on the yeast cell surface determine resistance to osmotin, a plant antifungal protein. Proc. Natl. Acad. Sci. USA. 1997;94:7082–7087. doi: 10.1073/pnas.94.13.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Teparic R., Stuparevic I., Mrsa V. Increased mortality of Saccharomyces cerevisiae cell wall protein mutants. Microbiology. 2004;150:3145–3150. doi: 10.1099/mic.0.27296-0. [DOI] [PubMed] [Google Scholar]
- 112.Kapteyn J.C., Hoyer L.L., Hecht J.E., Müller W.H., Andel A., Verkleij A.J., Makarow M., Van Den Ende H., Klis F.M. The cell wall architecture of Candida albicans wild-type cells and cell wall defective mutants. Mol. Microbiol. 2000;35:601–611. doi: 10.1046/j.1365-2958.2000.01729.x. [DOI] [PubMed] [Google Scholar]
- 113.Boorsma A., De Nobel H., ter Riet B., Bargmann B., Brul S., Hellingwerf K.J., Klis F.M. Characterization of the transcriptional response to cell wall stress in Saccharomyces cerevisiae. Yeast. 2004;21:413–427. doi: 10.1002/yea.1109. [DOI] [PubMed] [Google Scholar]
- 114.Martinez A.I., Castillo L., Garcera A., Elorza M.V., Valentın E., Sentandreu R. Role of Pir1 in the construction of the Candida albicans cell wall. Microbiology. 2004;150:3151–3161. doi: 10.1099/mic.0.27220-0. [DOI] [PubMed] [Google Scholar]
- 115.Jaafar L., Moukadiri I., Zueco J. Characterization of a disulfide-bound Pir-cell wall protein (Pir-CWP) of Yarrowia lipolytica. Yeast. 2003;20:417–426. doi: 10.1002/yea.973. [DOI] [PubMed] [Google Scholar]
- 116.Kauffman C.A., Carver P.L. Update on echinocandin antifungals. Semin. Respir. Crit. Care Med. 2008;29:211–219. doi: 10.1055/s-2008-1063859. [DOI] [PubMed] [Google Scholar]
- 117.Kurtz M.B., Rex J.H. Glucan synthase inhibitors as antifungal agents. Adv. Protein Chem. 2001;56:423–475. doi: 10.1016/s0065-3233(01)56011-8. [DOI] [PubMed] [Google Scholar]
- 118.Lozančić M., Hossain A.S., Mrša V., Teparić R. Surface Display—An Alternative to Classic Enzyme Immobilization. Catalysts. 2019;9:728. doi: 10.3390/catal9090728. [DOI] [Google Scholar]