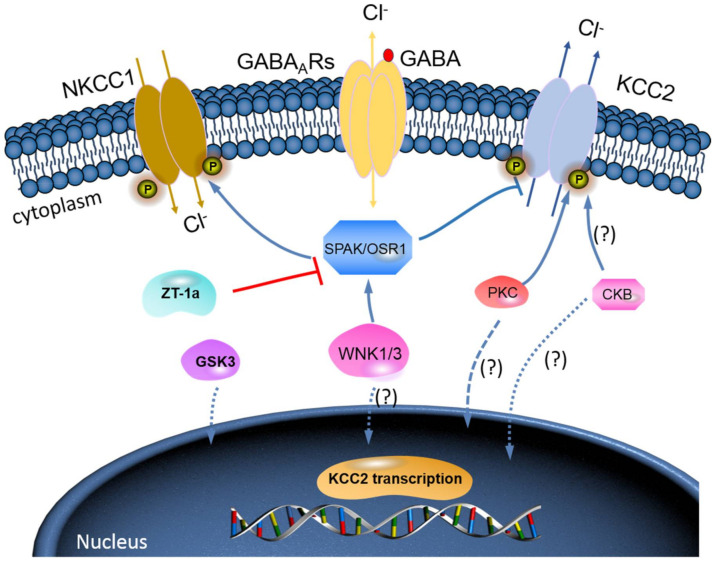
Figure 3.
A novel strategy to facilitate neuronal Cl− extrusion and EGABA by coincident NKCC1 inhibition and KCC2 activation by inhibiting the WNK-SPAK/OSR1 kinases. Mammalian neurons that are challenged with multiple neuropsychiatric conditions (such as seizures, neuropathic pain, spasticity, schizophrenia, and others) are usually driven by hyperexcitable circuits, intraneuronal Cl− ([Cl−]i) levels are elevated due to increased NKCC1 activity, and/or decreased KCC2 activity, promoting GABAAR-mediated membrane depolarization and excitation. In healthy mature neurons, [Cl−]i is low due to the opposite activity profile of the CCCs, promoting GABAAR-mediated hyperpolarization, which is critical for the proper balance of excitation–inhibition in neuronal circuits. WNK-SPAK/OSR1 inhibition, via the coincident effects of NKCC1 inhibition and KCC2 activation (the main Cl− extrusion mechanism in neurons), might be a potent way of facilitating neuronal Cl− extrusion to restore ionic inhibition in diseases that are characterized by disordered Cl− homeostasis and GABA disinhibition. ZT-1a, a novel molecular compound, can specifically inhibit SPAK signalling pathway, thus interfering SPAK regulation of GABA signalling via NKCC1 inhibition and KCC2 activation [86]. Activation of protein kinase C (PKC) and brain-type creatine kinase (CKB) are likely to increase KCC2 cell surface expression, but the mechanisms involved are still unclear.