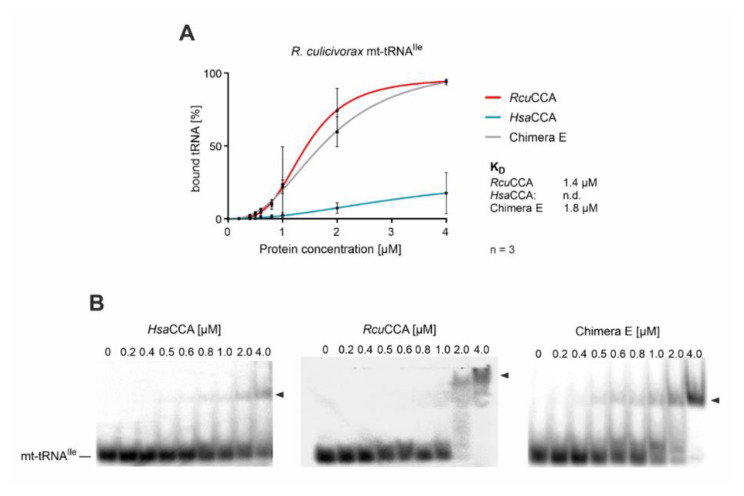
Figure 4.
Binding of wt and chimeric CCA-adding enzymes to an armless tRNA. Quantitative analysis of enzyme binding to the armless mt-tRNAIle determined by electrophoretic mobility shifts. (A) While the tRNA interaction of HsaCCA over the whole concentration range (0–4 µM) is too weak to calculate dissociation constants, RcuCCA as well as chimera E exhibit a strong affinity to this substrate, resulting in dissociation constants of 1.4 and 1.8 µM, respectively. Data are means ± SD; n = 3. (B) Images of representative gel shift assays on HsaCCA, RcuCCA, and chimera E with mt-tRNAIle as substrate.