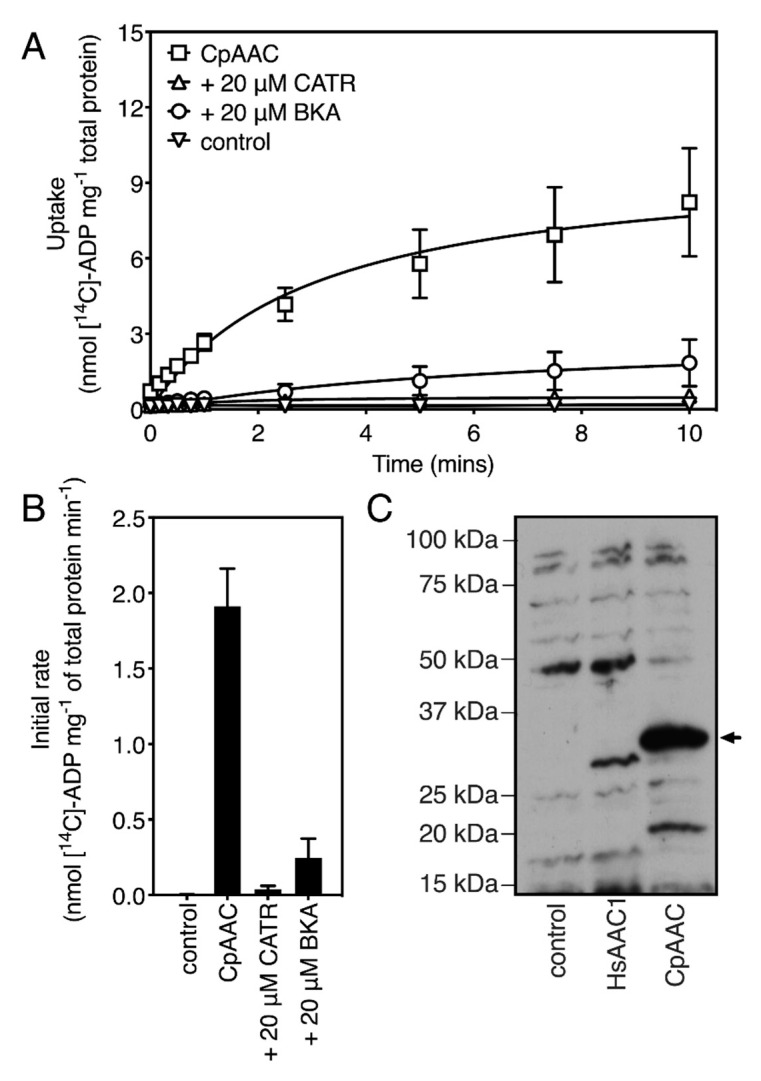
Figure 4.
CpAAC expressed in Lactococcus lactis retains functional characteristics of the mitochondrial ADP/ATP carriers. (A) ADP transport by CpAAC can be inhibited by carboxyatractyloside (CATR) and bongkrekic acid (BKA), the canonical inhibitors of mitochondrial AACs. Fused membrane vesicles of lactococcal membranes expressing CpAAC were preloaded with 5 mM ADP in the absence (squares) or presence of 20 μM CATR (triangles) or BKA (circles). The empty vector controls are shown as inverted triangles. Transport was initiated by the addition of 1.5 μM [14C]-ADP; (B) The specific initial transport rates, when the applied chemical gradients of radio-labeled and cold substrates are maximal, are derived from the linear parts of the uptake curves, typically in the first minute, corrected for the amount of protein. The data are represented by the mean and standard deviation of two biological repeats, each performed in quadruplicate; (C) Western blot of cytoplasmic membranes of L. lactis strains expressing CpAAC or human AAC1 (HsAAC1), which was characterized previously by [32], or transformed with empty vector. The band of CpAAC (approximately 33 kDa) was detected with a chicken antibody raised against the antigen YPLDTVRRRMMMT and anti-chicken-horseradish peroxidase conjugate. It is marked with an arrow. 10 µg of total protein was loaded per lane.