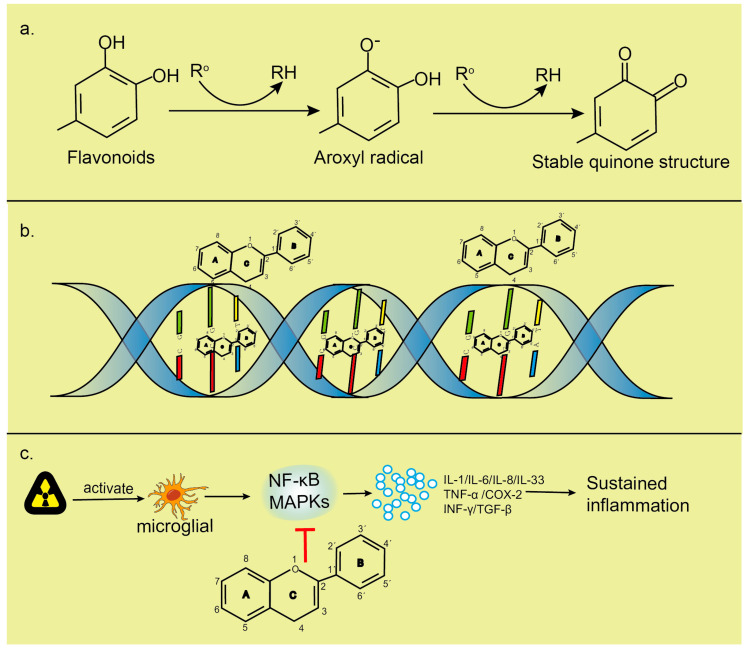
Figure 4.
(a). Flavonoids are able to reduce highly oxidizing free radicals with redox potentials, such as superoxide, peroxyl, alkoxyl and hydroxyl radicals, by hydrogen atom donation. Ro represents superoxide anion, peroxyl, alkoxyl and hydroxyl radicals. The aroxyl radical may react with a second radical, acquiring a stable quinone structure. (b). Intercalation of flavonoids into DNA double helices induces stabilization of DNA helical structures and condensation of DNA to a highly compact form that is less susceptible to attacks by free radicals; flavonoids can interact with the phosphate moiety of the DNA backbone through hydrogen bonding. The repair of sugar radicals is attributed to hydrogen donation from flavonoids through this bonding. (c). Flavonoids inhibit the activation of NF-κB and MAPK, reduce the release of inflammatory factors and play an anti-inflammatory role.