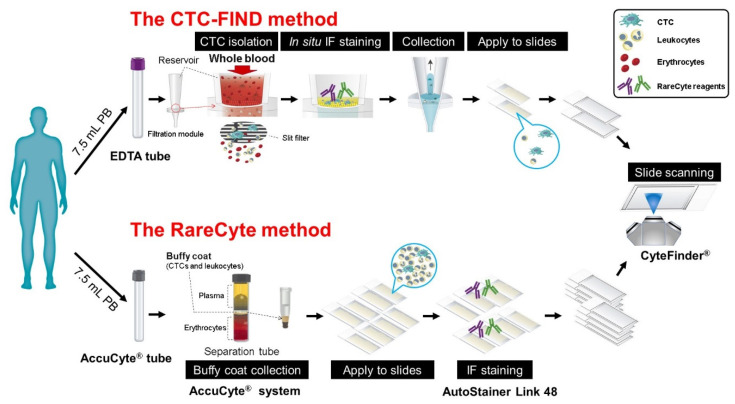
Figure 6.
Schematic diagram of the study design for the counting of CTCs. A schematic diagram of the techniques used by the CTC-FIND method and the RareCyte method. Peripheral blood from each cancer patient was collected into two collection tubes: an ethylenediaminetetraacetic acid (EDTA) tube for the CTC-FIND method and an AccuCyte® blood collection tube for the RareCyte method. The CTC-FIND method uses a slit-filter membrane for the isolation of CTCs, and CTCs were isolated from 7.5 mL of whole blood. After filtering, the trapped cells, including CTCs and leukocytes, were then subjected to in situ staining and collected into a 1.5 mL tube. Next, these cells were spread onto two positively charged slides, followed by the identification and enumeration of the CTCs using CyteFinder®. In the RareCyte method, the AccuCyte® system was used to separate nucleated cells in the buffy coat, including the CTCs and leukocytes, from 7.5 mL of whole blood. The resulting population of nucleated cells was then spread onto eight positively charged slides and immunostained using an AutoStainer Link 48. The CTCs were scanned and identified by CyteFinder® to enumerate them. CTCs were analyzed in 21 prostate cancer patient blood samples, nine bladder cancer patient blood samples, three kidney cancer patient blood samples, and three pancreatic cancer patient blood samples by using each method.