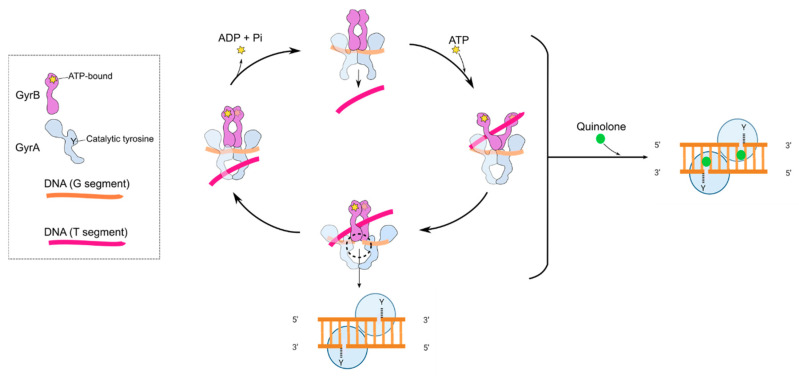
Figure 4.
Cartoon showing the proposed mechanism of DNA supercoiling by DNA gyrase and how quinolones interfere with this mechanism by stabilising the cleavage complex. The inset shows the GyrA (blue) and GyrB (purple) subunits. Y indicates the position of the active site tyrosine, and the star indicates the position of the ATP-binding site. The G segment (orange) binds across the GyrA dimer interface. The GyrA C-terminal domain wraps the DNA (not shown) to present the T segment (pink) in a positive node. ATP binds to the N-terminal domain of GyrB, which closes the GyrB clamp (also known as the N-gate), capturing the T segment. The G segment is transiently cleaved, the GyrB domains rotate (not shown), the DNA gate widens and the T segment is transported through the cleaved G segment. The G segment is re-ligated, and the T segment exits through the GyrA C-gate. The hydrolysis of ATP and the leaving of ADP + Pi resets the enzyme for another cycle, although the exact timing of these reactions is unknown. The black-dashed circle and lower inset show the cleavage complex. The right-hand panel shows the binding of quinolones (green spheres) in the cleavage complex.