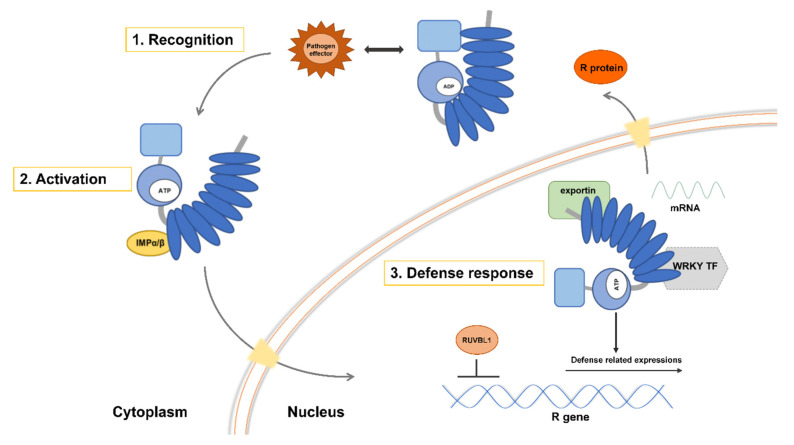
Figure 6.
Putative model for defense activation by NLRs in host plant triggered by parasitic plant. Induction of defense responses by NB-LRRs (nucleotide-binding site domain and a leucine-rich repeat domain) proceeds in three stages. In some cases, kinase will take part in indirect recognition. In the first stage, the inactive NLR receptor (blue) perceives the presence of specific virulence proteins, called pathogen effector (brown), secreted from parasitic plant, then binds with pathogen effector. In some cases, kinase (also referred as host factor) will take part in indirect recognition. NB-LRR could indirectly recognize pathogen effector through N-terminal domain (CC or TIR) by an intermediary kinase. In the second stage, NB-LRR receptor is activated by a conformation change and ATP binding to NB domain. A highly conserved nucleotide-binding domain that is shared with apoptotic protease activating factor 1 (APAF-1), various R-proteins, and CED-4 (NB-ARC domain) is proposed to act as a molecular switch, cycling between ADP (repressed) and ATP (active) bound forms [132]. In the third stage, activated NB-LRR work in the nucleus to induce defense-related signaling and gene expression. NLR negative regulators of defense such as (TIP49a) transcription factor (TF) is inhibited. Alternatively, WRKY transcription factor (TF) may bind to NLR to positively regulate and induce defense expression. Chimeric proteins comprise domains typical for both intracellular type-R proteins (NBS–LRR proteins) and WRKY transcription factors [146], suggesting that WRKY TF binds to NLR closely. To cross the nuclear pore, NLRs with a classical nuclear localization signal will require importin-α and importin-β (light yellow) for import and export [104]. Last, specific defense-related mRNAs or proteins are exported through nuclear pore. IMPα/β: importin-α/β; R genes: RPS5.