Scheme 2.
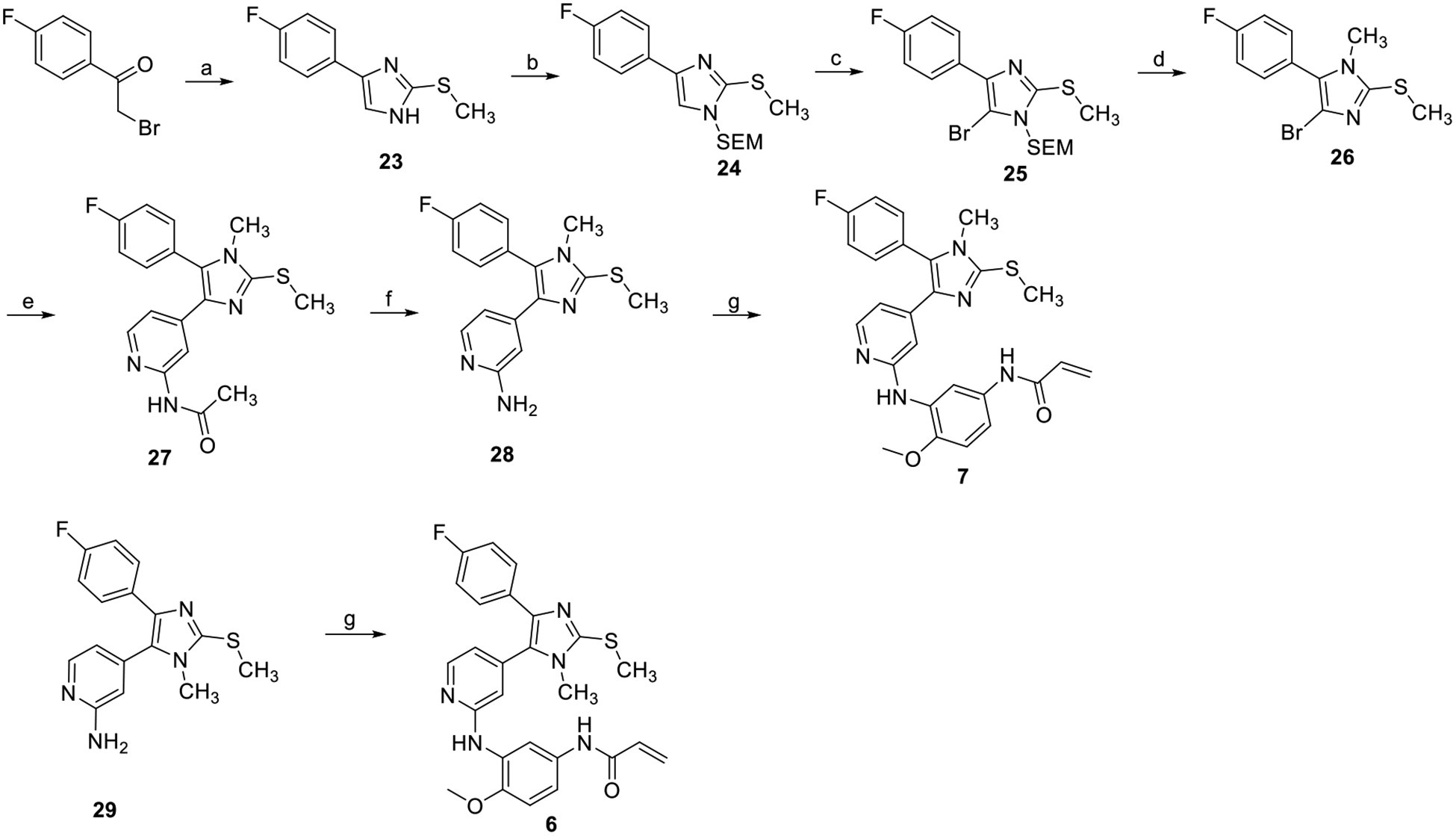
Reagents and conditions: (a) S-methylisothiourea, NaHCO3, THF/water, reflux (50%); (b) NaH, SEM-Cl, THF, 0°C to rt (91%); (c) NBS, THF, −30°C (85%); (d) MeOtf, DCM, TFA, rt (70%); (e) N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)acetamide, P(t-bu)3 Pd G3, K3PO4, 1,4-dioxane/water, 50°C (39%); (f) NaOH, MeOH/water, 50°C (88%); (g) N-(3-bromo-4-methoxyphenyl)acrylamide, Brettphos Pd G3, Cs2CO3, t-BuOH/1,4-dioxane, rf (67%).
