Abstract
BACKGROUND & AIMS:
Noninvasive tests used in colorectal cancer screening, such as the fecal immunochemical test (FIT), are more acceptable but detect neoplasias with lower levels of sensitivity than colonoscopy. We investigated whether lowering the cut-off concentration of hemoglobin for designation as an abnormal FIT result increased the detection of advanced neoplasia in a mailed outreach program.
METHODS:
We performed a prospective study of 17,017 uninsured patients, age 50 to 64 years, who were not current with screening and enrolled in a safety-net system in Texas. We reduced the cut-off value for an abnormal FIT result from 20 or more to 10 or more μg hemoglobin/g feces a priori. All patients with abnormal FIT results were offered no-cost diagnostic colonoscopy. We compared proportions of patients with abnormal FIT results and neoplasia yield for standard vs lower cut-off values, as well as absolute hemoglobin concentration distribution among 5838 persons who completed the FIT. Our primary aim was to determine the effects of implementing a lower hemoglobin concentration cut-off value on colonoscopy demand and yield, specifically colorectal cancer (CRC) and advanced neoplasia detection, compared with the standard, higher, hemoglobin concentration cut-off value.
RESULTS:
The proportions of patients with abnormal FIT results were 12.3% at the 10 or more μg hemoglobin/g feces and 6.6% at the standard 20 or more μg hemoglobin/g feces cut-off value (P = .0013). Detection rates for the lower vs the standard threshold were 10.2% vs 12.7% for advanced neoplasia (P = .12) and 0.9% vs 1.2% for CRC (P = .718). The positive predictive values were 18.9% for the lower threshold vs 24.4% for the standard threshold for advanced neoplasia (P = .053), and 1.7% vs 2.4% for CRC (P = .659). The number needed to screen to detect 1 case with advanced neoplasia was 45 at the lower threshold compared with 58 at the standard threshold; the number needed to scope to detect 1 case with advanced neoplasia increased from 4 to 5. Most patients with CRC (72.7%) or advanced adenoma (67.3%) had hemoglobin concentrations of 20 or more μg/g feces. In the group with 10 to 19 μg hemoglobin/g feces, there were 3 patients with CRC (3 of 11; 27.3%) and 36 with advanced adenoma (36 of 110; 32.7%) who would not have been detected at the standard positive threshold (advanced neoplasia Pcomparison < .001). The proportion of patients found to have no neoplasia after an abnormal FIT result (false positives) was not significantly higher with the lower cut-off value (44.4%) than the standard cut-off value (39.1%) (P = .1103).
CONCLUSIONS:
In a prospective study of 17,017 uninsured patients, we found that reducing the abnormal FIT result cut-off value (to ≥10 μg hemoglobin/g feces) might increase detection of advanced neoplasia, but doubled the proportion of patients requiring a diagnostic colonoscopy. If colonoscopy capacity permits, health systems that use quantitative FITs should consider lowering the abnormal cut-off value to optimize CRC detection and prevention. (ClinicalTrials.gov no: NCT01946282.)
Keywords: Colon Cancer, Tumor, Adenoma, Early Detection, Removal
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States. Screening can reduce CRC incidence and mortality,1–6 but participation is suboptimal among underserved populations, particularly minorities and the uninsured.2,7–10 Noninvasive tests for CRC screening such as the fecal immunochemical test (FIT) have been linked to higher participation rates,6,11–13 but have lower sensitivity for CRC and advanced adenoma than colonoscopy. Some FITs are quantitative and measure the absolute hemoglobin (Hgb) concentration within a stool sample. One strategy to improve neoplasia detection among individuals preferring FIT would be to decrease the Hgb concentration that would indicate an abnormal test result requiring diagnostic colonoscopy. Prior work has suggested that a lower threshold can improve neoplasia detection; a meta-analysis of FIT performance found that using a lower hemoglobin concentration cut-off value to designate an abnormal FIT optimized sensitivity for CRC: 89% for a cut-off value of less than 20 μg hemoglobin/g feces vs 70% for cut-off values of 20 to 50 μg hemoglobin/g feces.14 A challenge of this approach, however, particularly in settings with constrained colonoscopy capacity, is that decreasing the threshold also may increase colonoscopy demand. In the United States in particular, real-world data specific to the impact of using a lower Hgb concentration threshold for diagnostic colonoscopy demand and neoplasia detection when implemented within screening programs are sparse.
As part of a large mailed FIT outreach program, we applied a lower Hgb concentration cut-off value to define abnormal FIT. The aim of this analysis was to understand the impact of implementing a lower Hgb concentration cut-off value on colonoscopy demand and yield, specifically CRC and advanced neoplasia detection, compared with the standard higher Hgb concentration cut-off value routinely used to trigger diagnostic colonoscopy within an organized outreach screening program.
Methods
Study Population and Setting
The study includes participants from a comparative effectiveness trial performed at John Peter Smith Health Network (JPS), offering financial incentives to increase participation in CRC screening using a mailed invitation to complete FIT, as described in detail previously.15 Briefly, 17,017 patients ages 50 to 64 who were not up to date with CRC screening were identified within JPS, the safety-net health system for Tarrant County, Texas, and invited to complete FIT through mailed outreach.15 Patients who were not up to date with screening were defined by the absence of a documented colonoscopy in the past 10 years, sigmoidoscopy in the past 5 years, or stool occult blood testing in the past year, and included a mix of patients with and without prior exposure to any screening test. JPS includes a large public hospital and network of more than 60 community clinics, and offers a medical assistance program, JPS Connection, for uninsured individuals in need of medical care with insufficient financial resources residing in Tarrant County as a US citizen or legal permanent resident. As a result, all participants in the study were uninsured and enrolled in JPS Connection. Patients were excluded if they had a history of inflammatory bowel disease, CRC, or colon resection; no address/telephone number on file; or were incarcerated at the time of the study. Patients with a normal FIT (hemoglobin concentration, <10 μg hemoglobin/g feces) result after the first response to mailed outreach were re-invited to participate in screening the next year, along with patients newly eligible for participation, typically either by age or enrollment in the JPS Connection program. Outreach took place between November 2014 and December 2016.
Intervention and Follow-Up Evaluation
Organized outreach consisted of the following: (1) invitation in English and Spanish to use and return a FIT; (2) an enclosed 1-sample Polymedco (Cortland Manor, NY) OC Sensor FIT test; (3) 2 automated telephone reminders in English and Spanish to encourage test completion, delivered at the time of invitation and 1 week later; and (4) up to 2 live telephone reminders attempted within 4 weeks after invitation if screening was not completed or the patient was not reached during the initial call attempt. Completed kits were analyzed using the OC-Auto Micro 80 Analyzer. The threshold for defining an abnormal result was set to 10 μg or more hemoglobin/g feces a priori, a level lower than the more than 20 μg hemoglobin/g feces cut-off value recommended by the test manufacturer; these values correspond to 50 and 100 ng hemoglobin/dL buffer, respectively.
Patients with an abnormal FIT were contacted by telephone and mail to arrange for a no-cost diagnostic colonoscopy, and subjects with normal FIT were referred back to the screening program for continued participation.
Definitions
For the purpose of this study, we evaluated outcomes by hemoglobin threshold, comparing the lower 10 μg hemoglobin/g feces cut-off value with the standard 20 μg hemoglobin/g feces threshold. These outcomes include rates for test positivity, detection of advanced neoplasia (AN), positive predictive value (PPV), as well as the number needed to screen and the number needed to scope to detect 1 case with AN. We defined positivity rate as the proportion of test completers with an abnormal FIT, and detection rate referred to patients with AN or CRC relative to all patients with a completed test. AN included CRC and advanced adenomas; advanced adenoma was defined as an adenoma 1 cm or larger, with villous or tubulovillous features, and/or high-grade dysplasia. Patient classification was mutually exclusive, such that patients with multiple findings were classified according to the most advanced diagnosis. PPV accounted for all patients diagnosed with AN or CRC proportional to patients with a positive FIT who also completed colonoscopy. The number needed to screen reflects the number of completed tests needed to find 1 patient with AN, and the number needed to scope is the number of colonoscopies needed to find identify 1 patient with AN.
Analytic Approach
Descriptive statistics were used to characterize the study population. Equality of Hgb concentration distribution across colonoscopy finding groups was tested using the Kruskal–Wallis test, and proportions were compared using the Pearson chi-square test. For all comparisons, statistical significance was determined using a P value less than .05 as the threshold; all P values were 2-sided. Analyses were performed using R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria). All authors had access to the study data and reviewed and approved the final manuscript.
Ethical Approval
A waiver of informed consent was approved for the study from the University of Texas Southwestern Medical Center (STU 082012–086) and JPS (110512.007f) Institutional Review Boards. A copy of the original approved protocol is available upon request. The study also is registered at ClinicalTrials.gov (NCT01946282).
Results
Study Population
The study profile is summarized in Figure 1. Between November 2014 and December 2016, a total of 17,017 patients were invited to participate in CRC screening through mailed outreach using FIT. Among invitees, 31.6% (n = 5383) completed at least 1 FIT, with 3763 completing multiple rounds over the course of the study period. These participants were diverse, with 62.3% female; 37.6% white, 28.3% black, 32.9% Hispanic, 2.4% Asian, and 29.8% other; and had a median age of 56 years (Table 1). A total of 1185 (22.0%) participants had an abnormal FIT at the reduced cut-off concentration (≥10 μg hemoglobin/g feces); of these, 640 (54.0%) completed a colonoscopy.
Figure 1.
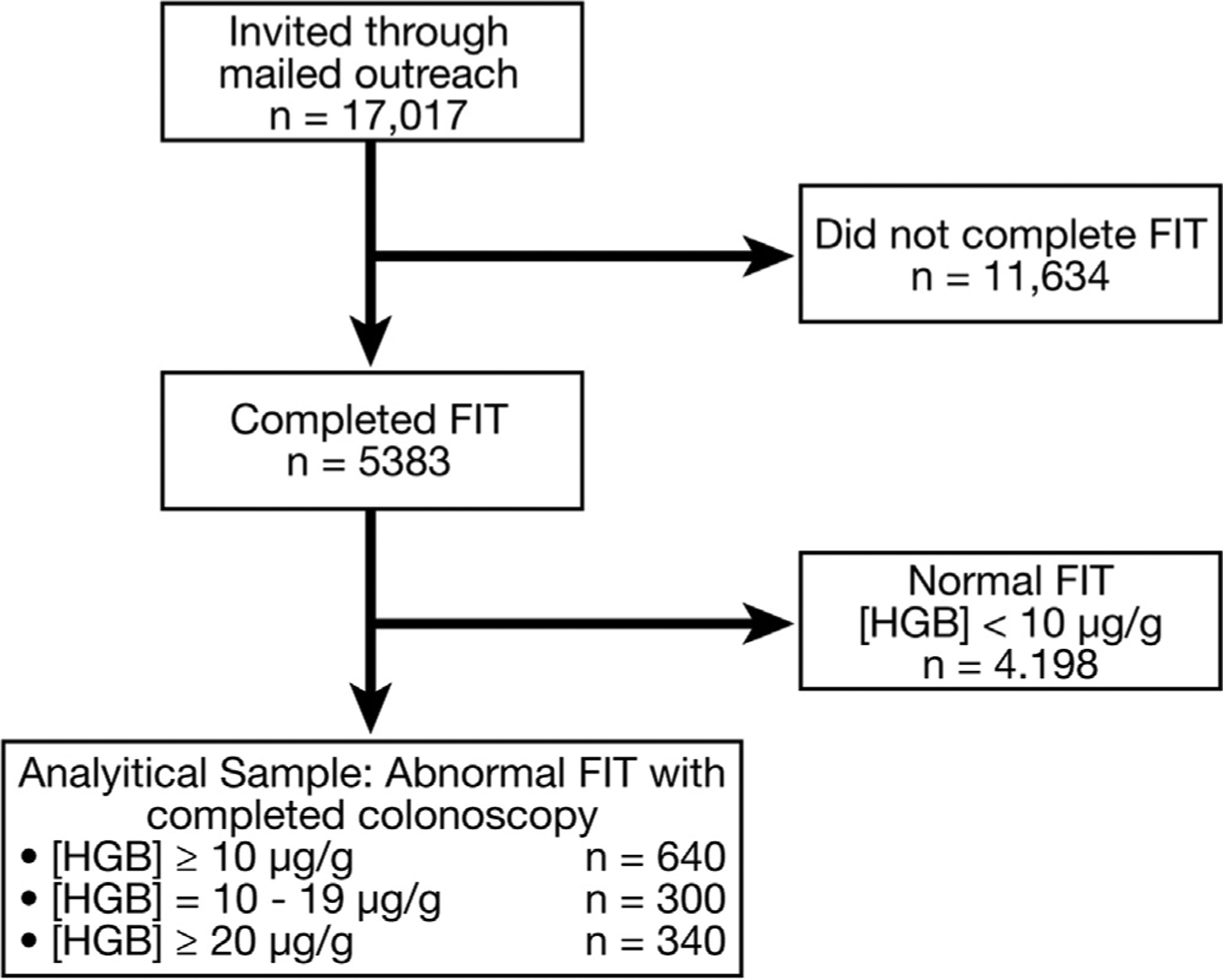
Study profile. FIT, fecal immunochemical test; Hgb, hemoglobin.
Table 1.
Characteristics of Patients With Abnormal FIT, Stratified by Colonoscopy Completion Status
| Abnormal FIT (n = 1185) | |||
|---|---|---|---|
| Overall | Colonoscopy completed | Colonoscopy not completed | |
| n (%) | 1185 (-) | 640 (54.00%) | 545 (45.99%) |
| Median age (IQR) | 57 (7) | 57 (7) | 58 (7) |
| Sex, n (%) | |||
| Female | 750 (63.29%) | 414 (64.59%) | 335 (61.47%) |
| Race, n (%) | |||
| White or Caucasian | 526 (44.39%) | 274 (42.81 %) | 252 (46.24%) |
| Black or African American | 332 (28.02%) | 192 (30.00%) | 141 (25.87%) |
| Other | 289 (24.39%) | 149 (23.28%) | 139 (25.50%) |
| Asian | 17 (1.43%) | 11 (1.72%) | 6 (1.10%) |
| Unknown | 17 (1.43%) | 12 (1.88%) | 5 (0.92%) |
| American Indian or Alaska Native | 3 (0.25%) | 1 (0.16%) | 2 (0.37%) |
| Native Hawaiian or other Pacific Islander | 1 (0.08%) | 1 (0.16%) | 0 (0.00%) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 309 (26.08%) | 151 (23.59%) | 158 (28.99%) |
| Not Hispanic or Latino | 853 (71.98%) | 472 (73.75%) | 382 (70.09%) |
| Unknown | 23 (1.94%) | 17 (2.66%) | 5 (0.92%) |
FIT, fecal immunochemical test; IQR, interquartile range.
Outcomes by Hemoglobin Threshold
The Hgb concentration ranged from 0 to 1560 μg hemoglobin/g feces, with a median Hgb concentration of 0.2 μg hemoglobin/g feces for patients with a normal FIT (interquartile range, 0.0–2.6). Demographic characteristics also were consistent between completers and noncompleters (Table 1).
Outcomes by Hgb threshold are shown in Figure 2. The AN detection rate was improved modestly at the lower vs higher hemoglobin threshold, but did not reach statistical significance: detection rate of 12.7 vs 10.2 for AN (P = .12) and 1.2 vs 0.9% for CRC (P = .718) at the lower vs higher cut-off value. When comparing the reduced cut-off value with the standard threshold, PPV was modestly worse for the lower vs higher threshold, but did not reach statistical significance: 18.9% vs 24.4% (P = .053) for AN and 1.7% vs 2.4% for CRC (P = .659) for the lower vs higher threshold, respectively. The number needed to screen to detect 1 case with AN was 58 at the lower vs 45 at the higher Hgb concentration threshold, whereas the number of patients with an abnormal FIT needed to scope to detect 1 case with AN was 5 at the lower vs 4 at the higher Hgb concentration threshold.
Figure 2.
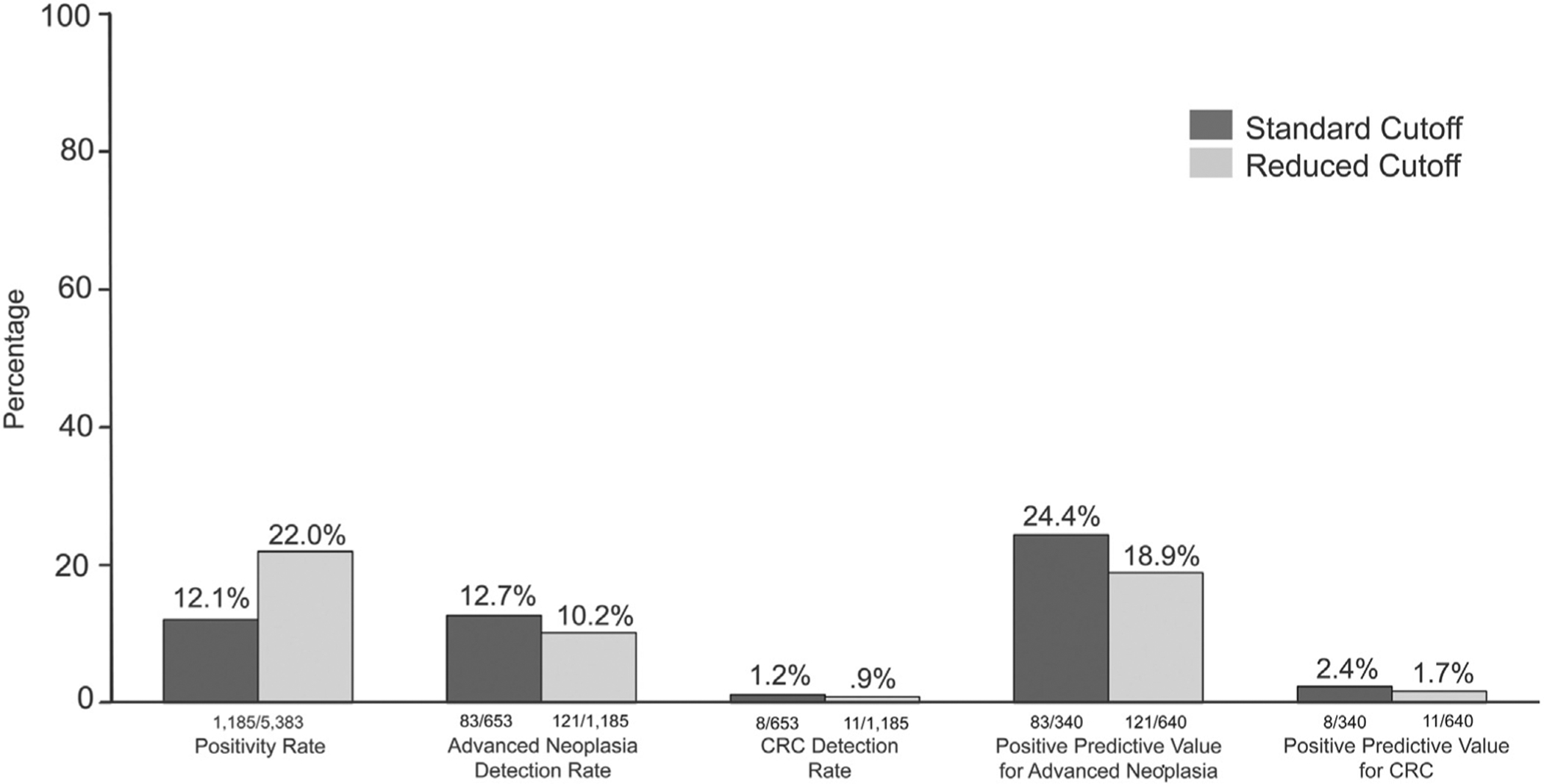
Outcomes by hemoglobin threshold. CRC, colorectal cancer.
Colonoscopy Findings by Hemoglobin Concentration
The median Hgb concentration was associated positively with severity of colonoscopy findings, ranging from 20.4 μg hemoglobin/g feces for individuals with no neoplasia to 121.8 μg hemoglobin/g feces for individuals with CRC (P trend = .0037) (Figure 3). Proportion of individuals with CRC and advanced adenoma identified only using the lower cut-off value vs the standard higher cut-off value was substantial: 27.3% of all individuals with CRC and 32.2% of all individuals with advanced neoplasia (Figure 4). Three individuals with CRC (Table 2) and 36 individuals with advanced adenoma would not have been recommended for colonoscopy if the standard 20 μg hemoglobin/g feces cut-off value had been used (P < .001 for comparison of low vs standard cut-off combined for advanced neoplasia). Although 50% of the 296 individuals with nonadvanced adenoma had a Hgb concentration of 10 to 19 μg hemoglobin/g feces, the increase in the proportion of patients with no neoplasia at colonoscopy was not significant (P = .11) when comparing the lower (44%) with the standard (39.1%) Hgb concentration threshold (P comparison = .1103). Of the 17 sessile serrated adenoma/polyps detected through colonoscopy, 9 (52.9%) had hemoglobin concentrations within the reduced cut-off range (10–19 μg hemoglobin/g feces), whereas 8 (47.1%) had hemoglobin concentrations at or above the standard cut-off value (>20 μg hemoglobin/g feces) range.
Figure 3.
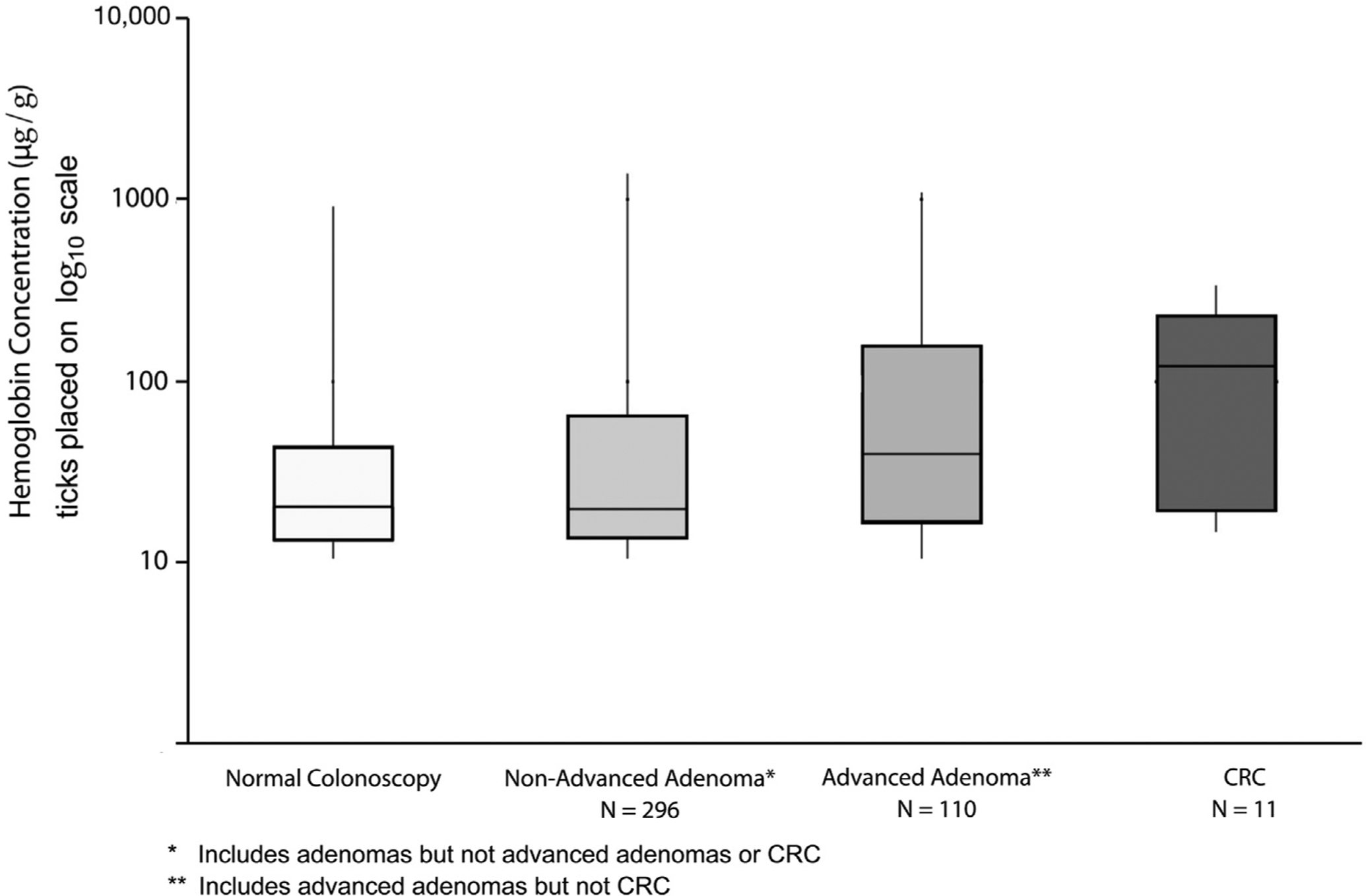
Distribution of hemoglobin concentration across colonoscopy findings. CRC, colorectal cancer.
Figure 4.
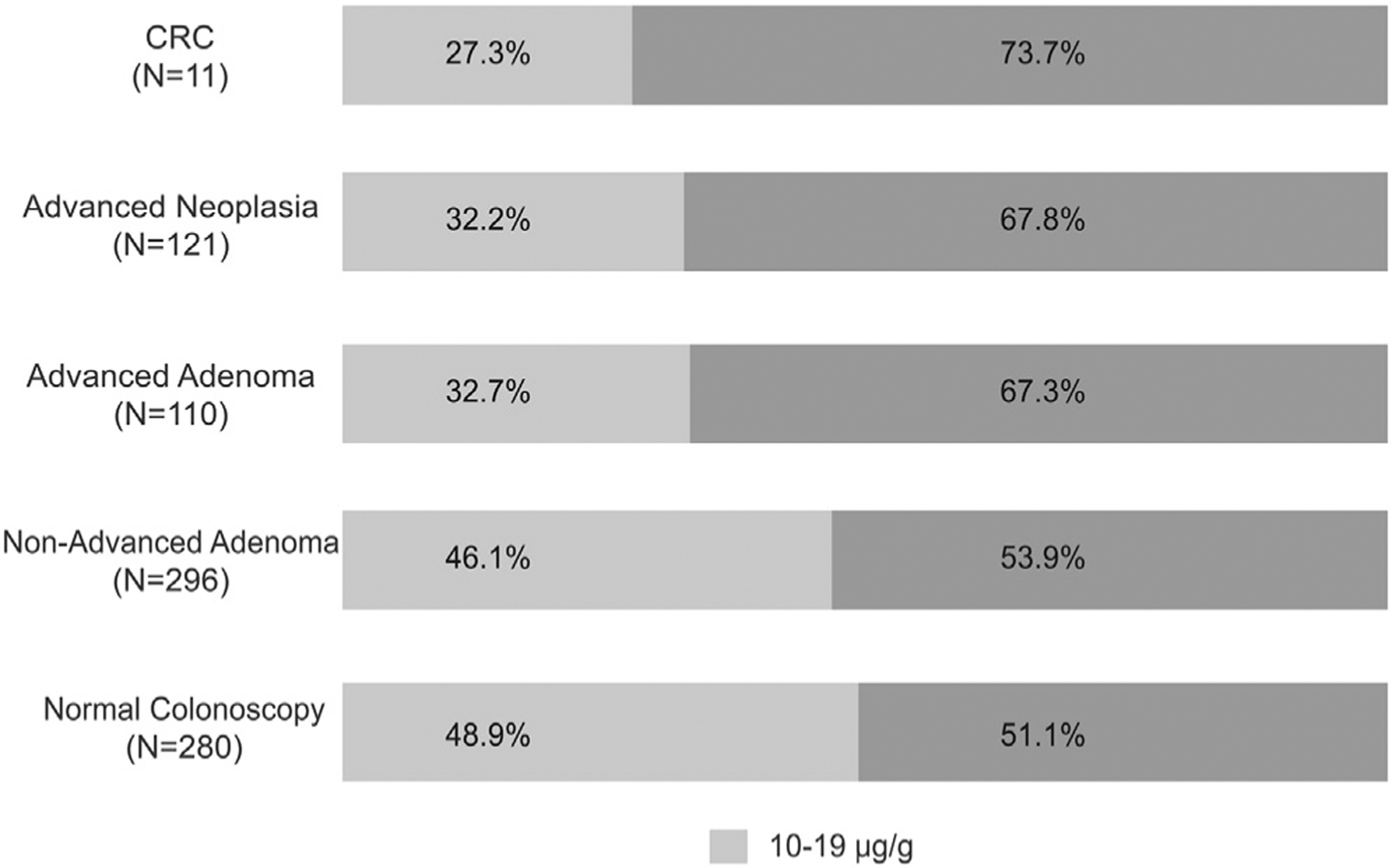
Proportion of patients with low vs standard cutoff abnormal FIT across colonoscopy outcomes. CRC, colorectal cancer.
Table 2.
Characteristics of Patients With CRC
| Patient ID | Hemoglobin concentration | Age, y | Sex | Location | Summary SEER stage |
|---|---|---|---|---|---|
| A | 15 | 61 | Female | Splenic flexure | Local |
| B | 16 | 56 | Female | Cecum | Regional |
| C | 17 | 57 | Female | Descending colon | Local |
| D | 22 | 53 | Female | Rectum | Local |
| E | 103 | 51 | Male | Sigmoid colon | Regional |
| F | 122 | 63 | Male | Sigmoid colon | Local |
| G | 133 | 63 | Female | Rectum | Local |
| H | 228 | 60 | Male | Rectum | Local |
| I | 232 | 61 | Male | Rectum | Local |
| J | 257 | 64 | Male | Rectum | Regional |
| K | 341 | 53 | Male | Rectum | Local |
SEER, Surveillance, Epidemiology, and End Results program.
Discussion
We found that implementing a lower Hgb concentration to define an abnormal FIT as part of a large outreach program may increase advanced adenoma and CRC detection compared with using the manufacturer-recommended standard threshold. Specifically, 1 in 3 patients with advanced adenoma and 3 of 11 patients with CRC would not have been detected by initial FIT screening at the standard threshold. Improvements to neoplasia detection came at the expense of increased colonoscopy demand. As a result, our results, together with prior work in this area, suggest that when colonoscopy capacity is adequate, lowering the cut-off threshold for quantitative FITs could improve advanced adenoma and CRC detection substantially as part of routine screening.
Our results confirm and extend prior literature documenting improved neoplasia yield when reduced hemoglobin thresholds are used, as well as the close relationship between increasing Hgb concentration and increasing neoplasia yield.16–21 This study analyzed the potential impact of using reduced cut-off values to improve neoplasia detection in the United States. Our results from a safety-net health system–based mailed outreach program in which a lower hemoglobin concentration threshold to trigger colonoscopy prospectively was implemented complement a recent retrospective study from a large integrated health system in California.21 In their usual-care FIT program, the standard threshold of more than 20 μg hemoglobin/g feces for the same OC-FIT CHEK (Cortland Manor, NY) test used in our program triggered referral for a diagnostic colonoscopy. For 640,859 patients, archived stool hemoglobin threshold results were identified, and programmatic CRC sensitivity (defined as identification of CRC by cancer registry or colonoscopy within 2 years of FIT) by hemoglobin concentration was estimated. Their primary finding was that programmatic CRC sensitivity increased at lower thresholds: 74.3% at the standard 20-μg hemoglobin/g feces threshold vs 79.3% at a reduced 10-μg hemoglobin/g feces threshold. They reported a very substantial increase in the number of patients with positive test results at the lower vs the standard threshold (84,293 vs 48,561), but estimated that only a modestly increased number of cancers would have been detected if a 10-μg hemoglobin/g feces threshold had been used (987 vs 925 cancers detected). Taken together, both studies suggest that lower thresholds could improve sensitivity for CRC in usual practice in the United States. Our study was complementary, in that all patients at the lower threshold were recommended colonoscopy, allowing the ability to estimate outcomes for CRC as well as advanced adenoma.
Quantitative FITs in the United States are approved and marketed as qualitative (abnormal/normal) tests. As such, in health care settings where quantitative FITs commonly are used, but with results reported only qualitatively as abnormal/normal at standard cut-off values, our data suggest an opportunity to improve early detection and prevention through disclosure of quantitative data. Specifically, quantitative data could be used as part of usual practice to decrease positivity thresholds that trigger diagnostic colonoscopy (thereby improving sensitivity for AN and CRC), and to triage urgency of colonoscopy for patients with higher quantitative values. We postulate that these strategies could be of special relevance when diagnostic colonoscopy capacity is sufficient to accommodate higher volume that comes with using lower thresholds.
Several potential limitations may be considered in interpreting our study. Because this study focused on an uninsured, generally low-income population, results may not be generalizable to all populations. Similarly, in our study only those patients with an abnormal FIT result were offered colonoscopy, and as a result missed lesions below this cut-off level could not be evaluated. Among patients with an abnormal FIT, 54% completed colonoscopy. Strategies proposed to improve colonoscopy completion in an underserved population have included patient navigation and cost coverage for clinical services, including transportation assistance.22,23 These methods were used as part of the study design, and yet patients with an abnormal FIT still experienced suboptimal colonoscopy completion rates. Additional research is needed to develop novel strategies to optimize colonoscopy follow-up evaluation, which could have as much or even greater impact on neoplasia detection as lowering the threshold to trigger diagnostic colonoscopy after an abnormal FIT. It is unknown whether the prevalence of neoplasia in people who completed vs did not complete colonoscopy differs and might have an impact on our estimates of neoplasia prevalence. Furthermore, the proportion of individuals with an abnormal FIT at the lower vs standard cut-off value who completed colonoscopy (56% vs 52%) might have impacted the precision of estimates of neoplasia detection and the ability to compare detection rates. Our comparison was nonrandomized; over multiple rounds of testing, outcomes among individuals for whom a 20-μg hemoglobin/g feces threshold was applied might have turned out to be similar to those for whom a lower threshold was applied owing to the detection of initially missed advanced neoplasia on subsequent rounds of testing. Our sample included 640 individuals who underwent a colonoscopy after an abnormal FIT; a larger sample could have allowed for more precise estimates of differences between outcomes stratified by FIT hemoglobin cut-off thresholds. These limitations are balanced by several strengths, including availability of a substantial sample of individuals with an abnormal FIT who completed colonoscopy, the inclusion of a diverse patient population, and presentation of data from a US population regarding outcomes of using differing thresholds for test positivity.
In conclusion, we found that implementing a lower cut-off value for an abnormal FIT as part of an organized mailed outreach program may result in the detection of additional patients with advanced neoplasia, although at the expense of doubling the proportion of patients requiring a diagnostic colonoscopy. Health systems using quantitative FIT should carefully consider the screening threshold before implementation, and if colonoscopy capacity permits, consider decreasing the cut-off value for an abnormal FIT as a potential strategy for optimizing CRC detection and prevention.
What You Need to Know.
Background
We investigated whether lowering the cut-off concentration of hemoglobin for designation as an abnormal fecal immunochemical test (FIT) result increased the detection of advanced neoplasia in a mailed outreach program.
Findings
In a prospective study of 17,017 uninsured patients, we found that reducing the abnormal FIT result cut-off value (to ≥10 μg hemoglobin/g feces) doubled the proportion of patients requiring a diagnostic colonoscopy, but increased detection of advanced neoplasia.
Implications for patient care
If colonoscopy capacity permits, health systems that use quantitative FITs should consider lowering the abnormal cut-off value to optimize colorectal cancer detection and prevention.
Acknowledgments
The authors thank the Polymedco Corporation for the loan of the fecal immunochemical test processing machine for this study.
Funding
This study was funded by PP120229 from the Cancer Prevention and Research Institute of Texas (Argenbright, PI). Also funded in part by National Cancer Institute/National Institutes of Health grant 5R37CA222866 (S.G.), and National Institutes of Health/National Cancer Institute grant P30 CA023100 (Lippman). The Cancer Prevention and Research Institute of Texas, National Cancer Institute, and National Institutes of Health had no role in the design, conduct, or reporting of the study.
Abbreviations used in this paper:
- AN
advanced neoplasia
- CRC
colorectal cancer
- FIT
fecal immunochemical test
- Hgb
hemoglobin
- JPS
John Peter Smith Health Network
- PPV
positive predictive value
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.American Cancer Society. Cancer facts and figures 2015; 2015:12–18. [Google Scholar]
- 2.Klabunde CN, Joseph DA, King JB, et al. Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morbid Mortal Wkly Report 2013;62:881. [PMC free article] [PubMed] [Google Scholar]
- 3.Kullgren JT, Dicks TN, Fu X, et al. Financial incentives for completion of fecal occult blood tests among veterans: a 2-stage, pragmatic, cluster, randomized, controlled trial. Ann Intern Med 2014;161:S35–S43. [DOI] [PubMed] [Google Scholar]
- 4.Purnell JQ, Thompson T, Kreuter MW, et al. Behavioral economics: “nudging” underserved populations to be screened for cancer. Prev Chronic Dis 2015;12:E06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Center for Quality Assurance. HEDIS measures for colorectal cancer screening; 2011.
- 6.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med 2013;173:1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Screening for Colorectal Cancer. US Preventive Services Task Force recommendation statement. Ann Intern Med 2008; 149:627–637. [DOI] [PubMed] [Google Scholar]
- 8.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570–1595. [DOI] [PubMed] [Google Scholar]
- 9.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116:544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klabunde C, Cronin KA, Breen N, et al. Trends in colorectal cancer test use among vulnerable populations in the US. Cancer Epidemiol Biomarkers Prev 2011;20:1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy, and fecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 2009;58:241–248. [DOI] [PubMed] [Google Scholar]
- 12.Walsh JM, Terdiman JP. Colorectal cancer screening: clinical applications. JAMA 2003;289:1297–1302. [DOI] [PubMed] [Google Scholar]
- 13.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014;160:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Miller S, Koch M, et al. Financial incentives for promoting colorectal cancer screening: a randomized, comparative effectiveness trial. Am J Gastroenterol 2016;111:1630. [DOI] [PubMed] [Google Scholar]
- 16.Hol L, Wilschut J, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer 2009;100:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieten E, Schreuders EH, Nieuwenburg SA, et al. Effects of increasing screening age and fecal hemoglobin cutoff concentrations in a colorectal cancer screening program. Clin Gastroenterol Hepatol 2016;14:1771–1777. [DOI] [PubMed] [Google Scholar]
- 18.Brenner H, Werner S. Selecting a cut-off for colorectal cancer screening with a fecal immunochemical test. Clin Transl Gastroenterol 2017;8:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aniwan S, Ek TR, Pongprasobchai S, et al. The optimal cut-off level of the fecal immunochemical test for colorectal cancer screening in a country with limited colonoscopy resources: a multi-center study from Thailand. Asian Pac J Cancer Prev 2017;18:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rossum L, Van Rijn A, Laheij R, et al. Cutoff value determines the performance of a semi-quantitative immunochemical faecal occult blood test in a colorectal cancer screening programme. Br J Cancer 2009;101:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selby K, Jensen CD, Lee JK, et al. Influence of varying quantitative fecal immunochemical test positivity thresholds on colorectal cancer detection: a community-based cohort study. Ann Intern Med 2018;169:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selby K, Baumgartner C, Levin TR, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med 2017;167:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med 2018;178: 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]


