Abstract
By recording neural activity directly from the human brain, researchers gain unprecedented insight into how neurocognitive processes unfold in real time. We first briefly discuss how intracranial electroencephalography (iEEG) recordings, performed for clinical practice, are used to study human cognition with the spatiotemporal and single-trial precision traditionally limited to non-human animal research. We then delineate how studies using iEEG have informed our understanding of issues fundamental to human cognition: auditory prediction, working and episodic memory, and internal cognition. We also discuss the potential of iEEG to infer causality through the manipulation or ‘engineering’ of neurocognitive processes via spatiotemporally precise electrical stimulation. We close by highlighting limitations of iEEG, potential of burgeoning techniques to further increase spatiotemporal precision, and implications for future research using intracranial approaches to understand, restore, and enhance human cognition.
Keywords: iEEG, ECoG, sEEG, iES, human cognition
1. Introduction to intracranial recording
The last decade has witnessed dramatic growth in the application of intracranial electroencephalography (iEEG)—that is, electrical activity recorded directly from the brains of awake, behaving humans—to address outstanding questions in the study of human cognition (figure 1(a)). These electrodes are implanted during surgery for clinical monitoring, usually to identify and/or prepare for resection of a focal epileptic source. Electrodes are placed subdurally in grids or strips to sample the cortex, as in electrocorticography (ECoG; figure 1(b)), and/or stereotactically into deeper structures to target the hippocampus and other subcortical regions (sEEG; figures 1(c), (d)). With inter-electrode spacing generally between 4–10 mm and sampling rates of 500–10 k Hz, macroelectrode ECoG/sEEG data afford both millimeter spatial precision and millisecond temporal precision.
Figure 1.
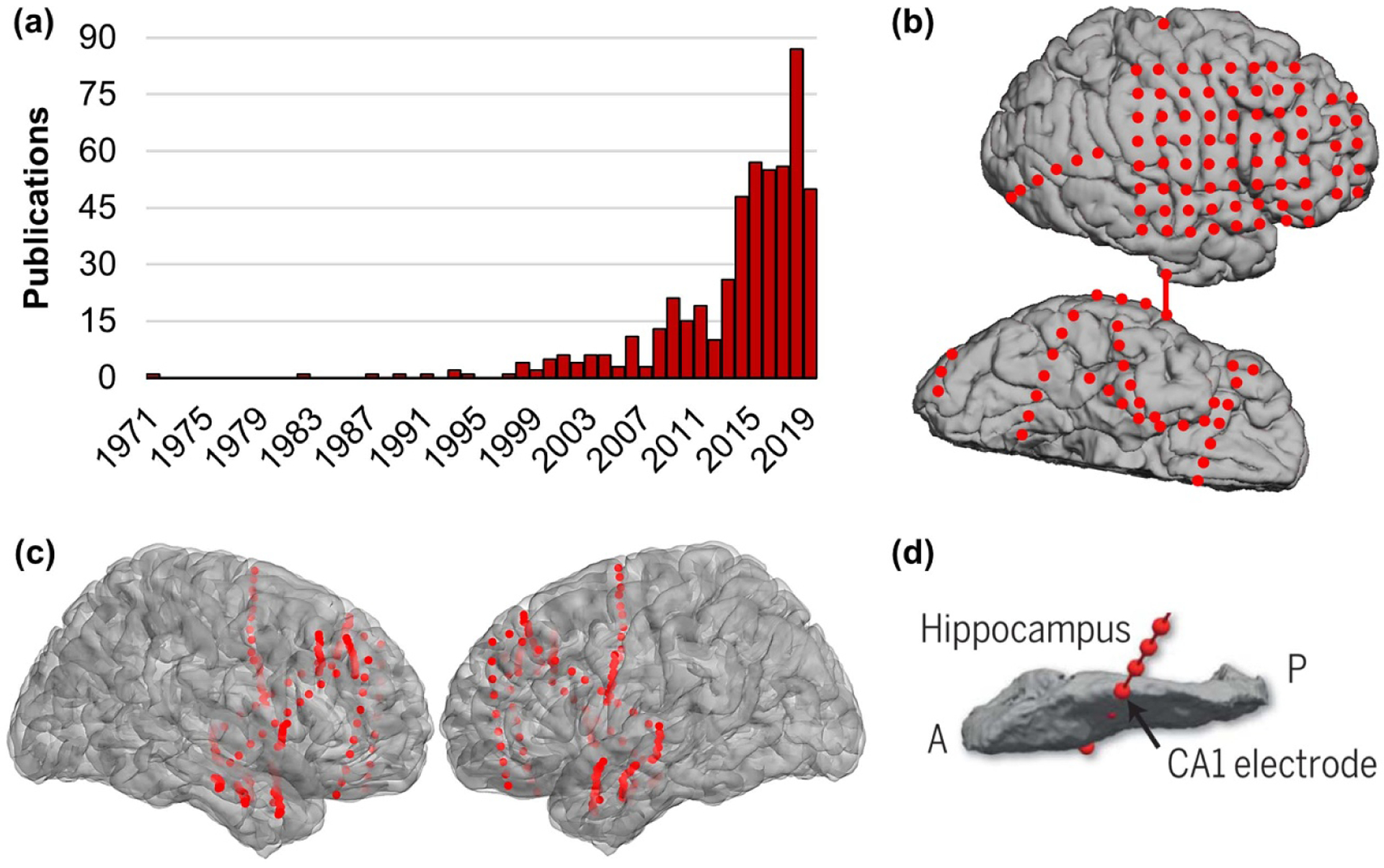
Intracranial research. (a) Number of publications in PubMed using the search terms ‘iEEG’ or ‘ECoG’ or ‘sEEG’ and ‘human cognition,’ 1971–2019. (b) Representative patient with right-hemisphere ECoG. Electrodes are shown on the lateral view and partial rendition of the ventral view. Red circles depict electrodes. (c) Representative patient with bilateral sEEG, same conventions as (b). (d) Schematic of sEEG targeting the hippocampus (CA1), same conventions as (b). A, anterior; P, posterior. Adapted from [1].
Approximately 1% of the human population is affected with epilepsy, and surgical management is recommended for medication-resistant patients to minimize the risks of neural dysfunction and premature death [2]. Although obtaining data from neurosurgical patients is not without logistical challenges [3], data are reliably collected during a range of experimentally controlled tasks. With appropriate cleaning to remove pathologic data related to seizures (e.g. data from channels in the seizure onset zone, epochs containing epileptic activity), ECoG/sEEG data represent healthy neural activity [4]. Further, direct access to the brain circumvents the low signal-to-noise ratio issue of noninvasive scalp EEG. Without the skull, skin, hair, or distance between electrodes and the brain, ECoG/sEEG data offer single-trial reliability [5] and the resolution needed to measure spectral activity at both low and high frequencies. Of note, high-frequency activity (HFA; measured over a broadband frequency range between 70 and 300 Hz) tracks population-level neuronal firing [6–9] and blood oxygen level dependent (BOLD) responses in cortical [10–12] and subcortical [13] regions. ECoG/sEEG data thus afford the spatiotemporal precision and signal-to-noise ratio usually limited to studies of animal models.
In this review, we highlight insights obtained from studies using macroelectrode ECoG and sEEG to study fundamental higher-order cognitive processes in humans, from the perception of external stimuli to internally oriented thinking in the absence of external cues. We utilize three topics to showcase how iEEG has expanded our understanding of human cognition beyond what has been possible with noninvasive neuroimaging approaches. Section 2 covers auditory perception and prediction, section 3 covers working and episodic memory, and section 4 covers internal cognition. In section 5, we close with a discussion of the use of intracranial electrical brain stimulation (iES) as a tool to engineer the brain and infer the causality of a brain region and/or function to a specific cognitive process. In the concluding remarks, we discuss limitations of iEEG, with focus on the compromise between high spatiotemporal precision and sparse brain coverage, and describe research using burgeoning intracranial approaches (e.g. microelectrode neuronal recording) to gain further insights into human cognition.
2. Auditory regularity detection and predictions
In our daily lives, we are exposed to sensory events that very often follow repetitive rules. Thanks to these rules, we are able to anticipate future events, which are potentially beneficial, or dangerous and harmful to our lives, before they occur [14]. Predictive mechanisms are embedded as inherent properties and microcircuits of the cortex [15], as well as in intrinsic connectivity across brain regions, which typically supports a hierarchical organization [15]. This organization comprises cortical areas that are ‘low’ in a cortical hierarchy (i.e. sensory cortices) and ‘higher’ in the hierarchy (prefrontal cortex [PFC]), which maintain a generative model of the environment. Information flow from ‘high’ to ‘low’ areas is facilitated by feedback connections, carrying predictions about the state of the world [16], and by their inverse, feedforward connections, carrying prediction error signals (i.e. mismatch between predicted and received information), which may result in a model update [14].
One prevalent experimental model for studying how we detect environmental rules and form predictions is through deviance paradigms in the auditory modality [17]. In the simplest case of deviance paradigms, a rule is built by repeatedly presenting the same auditory stimulus (standard) at regular intervals. This rule can be occasionally violated by replacing the standard with a different (deviant) stimulus. In the auditory modality, neural responses to deviant minus standard sounds reflect a typical scalp EEG event-related potential (ERP) component, the so-called mismatch negativity (MMN), which appears as a negative deflection that onsets at around 120 ms over fronto-central electrodes [18].
Initial attempts aiming to localize the neural origin of the MMN component combined scalp EEG with functional magnetic resonance imaging (fMRI). These studies, based on noninvasive recordings, identified two distinct sources: the first is a bilateral source in the superior temporal gyrus (STG) and the second is a source in the right inferior frontal gyrus (IFG) [19]. Since the discovery of these sources, several noninvasive EEG studies have capitalized on recordings from STG and IFG, and have built upon STG-IFG interactions to test theories of deviance processing [20–22]. Although noninvasive EEG studies corroborated previous findings by implicating STG and IFG in deviance detection, it was the examination of deviance processing using iEEG that markedly advanced our understanding of the neural underpinnings of this cognitive process. In particular, early iEEG studies hinted at the existence of a distributed network underlying the detection of deviant events, including not only temporal and frontal regions, but also posterior cingulate and parietal lobe [23], hippocampus and amygdala [24]. Despite these early intriguing findings, subsequent studies continued to focus on testing claims from scalp EEG studies, and confirmed that temporal and frontal areas underlie detection of deviant events using intracranial ERPs (typically measured between 1 and 20 Hz) [25].
Some of the questions on the regularity detection network that iEEG recordings aim to answer are:
Does regularity detection rely on a two-node prefrontal-auditory cortex system or a more widespread network?
What is the specific contribution of each node in this network and how is information flow established?
How is activity within this network modulated by external factors (i.e. relating to task structure, such as predictability and sequence complexity) and internal factors (relating to the state of participants, such as attention or consciousness)?
2.1. Regularity detection network
Rosburg and colleagues recorded iEEG from a large cohort of 29 patients and reported evidence of temporal sources of MMN, which were located in distributed regions of temporal cortex across patients [25]. However, few patients exhibited deviance responses in frontal electrodes, providing only a tentative confirmation of deviance detection mechanisms in frontal cortex. The involvement of temporal cortex in deviance detection was confirmed by another study, examining both ERPs and HFA [26]. This study revealed that the superior temporal plane is the core generator of the N1 scalp ERP component, responding to sounds, with posterior Sylvian fissure regions also being sensitive to deviance, but it did not provide reliable evidence for frontal deviance detection. Deviance responses in temporal cortex were elicited in the form of both ERPs and HFA, at latencies typically reported for scalp auditory components (figure 2(a)). More recently, Phillips and colleagues provided evidence for STG and IFG sources, both with iEEG and magnetoencephalography (MEG) recordings, supporting the view that a two-node circuit underlies deviance detection [16]. Interestingly, both techniques showed bidirectional connections between STG and IFG, suggestive of feedforward and feedback connections during processing of deviant events.
Figure 2.
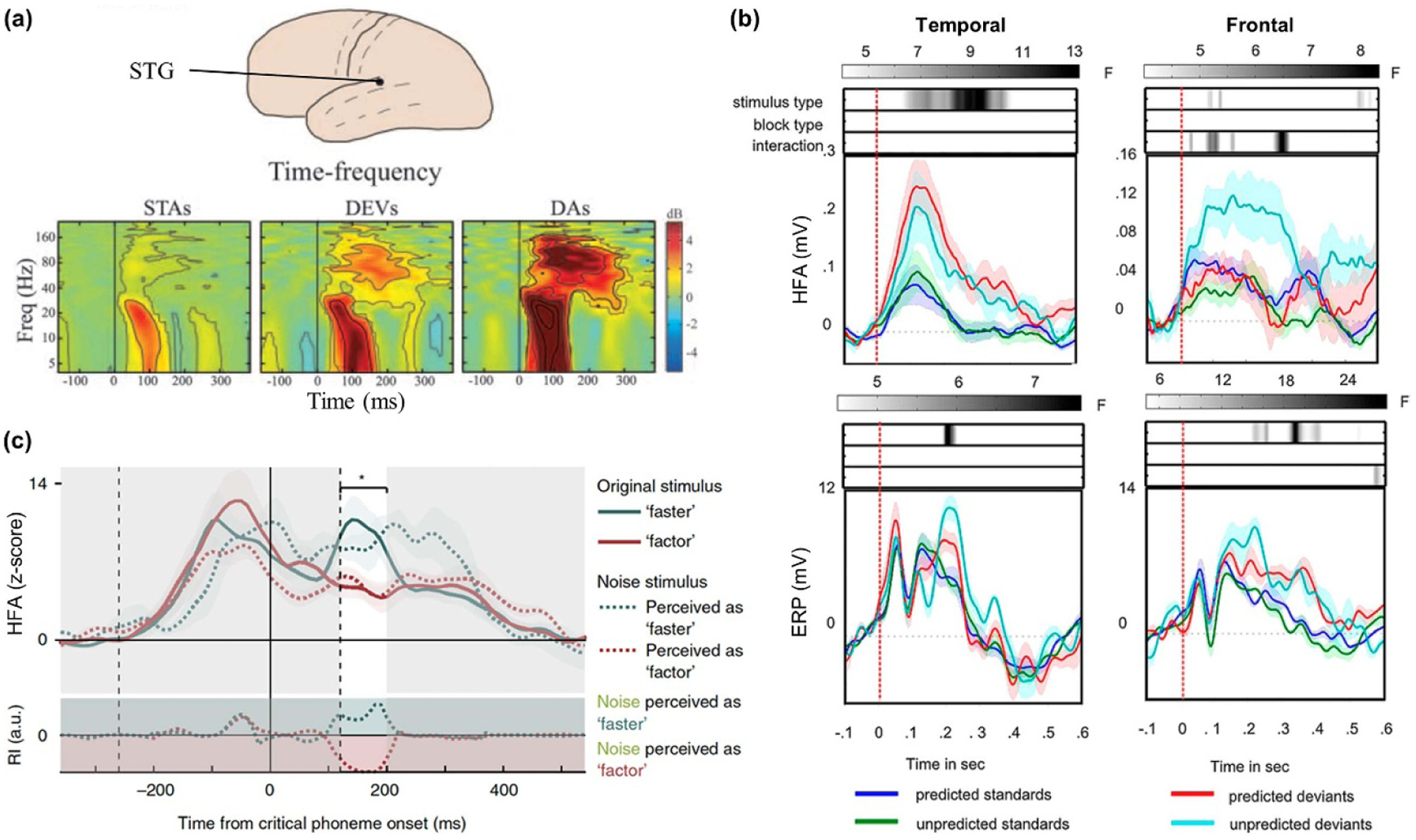
Auditory deviance detection and predictions. (a) Time-frequency analysis for an exemplar temporal electrode in response to standard (STAs) and deviant (DEVs) sounds, and a control condition of deviant sounds presented without any standards (DAs). An increase in power in response to standard and deviant sounds was observed for frequencies bellow 40 Hz and as early as 100 ms post-sound onset, with deviant sounds eliciting a stronger response than standard ones. Deviant sounds elicited an additional high-frequency response, between 40 and 160 Hz, between 35 and 350 ms. Adapted from [26]. (b) Deviance and predictability effects for frontal and temporal cortices. Temporal regions show deviance effects both for ERPs and HFA (bottom and top plots, respectively), but no interaction between deviance and predictability (shaded bars on top of each plot). By contrast, frontal areas show an interaction between deviance and predictability for HFA, suggesting sensitivity to auditory predictions. Adapted from [27]. (c) HFA in STG in response to auditory stimuli (full lines), or auditory stimuli that had a single phoneme replaced by noise (dashed lines). Responses between 100 and 200 ms post-stimulus onset to the noise stimuli resemble the responses to the original stimuli according to which they were perceived. RI (bottom plot) quantifies the relative distance between noise and original stimuli. Adapted from [28].
Apart from confirming the neural generators of scalp-level MMN, the use of iEEG has provided valuable insight about other locations that are sensitive to deviance detection and would otherwise remain unknown. Recent studies that capitalized on widespread electrode coverage have added new key regions in the deviance detection network [29] supporting a more distributed network of deviance processing. For example, Blenkmann and colleagues reported a strong deviance response in HFA in the human insula [29]. Importantly, these authors contrasted insular responses with responses from temporal areas, including STG and superior temporal sulcus (STS). Both areas showed earlier auditory responses compared to insula [29], suggesting a temporal hierarchy in the network of deviance detection. The full extent of the deviance network, and how each node therein is manipulated by different features of deviant sounds or levels of attention, remains to be explored in future iEEG studies.
2.2. Regularity detection over multiple timescales
Apart from assessing detection of auditory regularities at the level of single sounds, several groups have investigated auditory regularity detection over multiple timescales. A prevalent paradigm is the local-global paradigm, which contains embedded auditory regularities, formed by repetitions of single sounds (local) or groups (global) of sounds [30, 31]. Early studies using this paradigm claimed that a widespread network of brain regions is sensitive to violations of global rules, whereas a focal network is sensitive to violations of local rules [32]. Contrary to this initial evidence, further studies introduced a more systematic use of iEEG, including larger patient cohorts and more extensive coverage, and showed strong spatial overlap in regions that respond to violations of both local and global rules [30, 31]. This spatial overlap suggests that common mechanisms give rise to regularity detection over multiple timescales.
In particular, one study showed evidence for both local and global deviance responses in in STG and STS [30]. The authors also found that local deviance detection manifested at earlier latencies and in more widespread electrodes compared to global deviance detection. Interestingly, the global effect, but not the local one, was found in a subset of frontal electrodes, as indexed by ERPs and HFA. More recently, Nourski and colleagues reported a remarkable spatial overlap in temporal regions exhibiting both local and global effects [31]. This study extended previous findings [30] by reporting local effects in frontal electrodes in ERPs, but not HFA, while global effects in frontal electrodes were observed in both measures. Importantly, both local and global effects showed a hierarchical activation of brain regions, starting from core primary auditory areas, and progressing to noncore auditory and peripheral regions, including STG and PFC. Latencies for the local effect were on average shorter than for the global effect. Taken together, these studies challenge early views about the local effect recruiting a very focal network and the global effect relying on a widespread one [32]. Instead, they suggest that a common network is sensitive to violations of multi-scale auditory regularities, but at different latencies.
An important consideration regarding the detection of auditory regularities is the role of attention and conscious access to the environment. There is a plethora of evidence showing that, although regularity detection is modulated by attention, it can take place in an automatic way, even when attention is distracted, and in some cases even when conscious access to the environment is impaired [17]. The role of attention and conscious perception is particularly crucial for the local-global paradigm, as the presence of a global deviance response has been proposed as a marker of conscious perception and active attention [32].
An iEEG study challenged this view by examining how local and global deviance detection are affected by propofol-induced anesthesia and loss of consciousness [33]. In this study, local deviance detection effects were diminished but still present across all recorded regions during sedation, and were eventually restricted to auditory areas only (core and noncore) upon loss of consciousness. Frontal regions were the most sensitive to sedation, as they were first to show suppression of the local effect. This study reported that the spatial extent of the global effect was substantially suppressed in a sedated state during which some patients were still responsive, indicating that the loss of a global response is not directly linked to loss of consciousness [33].
In summary, local and global deviance effects manifest over a wide-spread network of brain regions and are modulated by the loss of consciousness. By providing increased signal-to-noise ratio and spatial sensitivity, intracranial recordings suggest that there is not a one-to-one correspondence between their presence/absence and consciousness. Although intriguing at first, the view that an automatic circuit that operates without explicit attention or consciousness underlies deviance detection over multiple scales is hardly surprising. Non-human animal electrophysiology and human EEG studies have shown evidence of automatic adaptation to auditory events over long timescales in anesthetized brains [34], evidence of implicit tracking of long auditory sequences to conditions of distracted attention [35], as well as the presence of a global effect in patients in a coma and under sedation [36]. Future iEEG studies can examine the limits in the automaticity of this circuit by employing experimental designs inducing regularities over longer timescales in combination with attentional and consciousness manipulations.
2.3. From regularity detection to auditory predictions
Deviance detection paradigms provide an indirect insight into the formation of auditory predictions. The underlying assumption is that, in order for a deviant event to be detected as such at a neural level, there must be first an identification of a standard event, and thus a low-level prediction. In other words, if we are exposed to the same stimulus multiple times, we can ‘predict’ that the next stimulus will be of the same type.
A typical paradigm for studying predictive processes is the omission response paradigm, in which a sequence is established by repeating a series of sounds, while some sounds are intermittently omitted and replaced by a silent period [37, 38]. Early studies showed that temporal electrodes respond not only to sounds, but also to their omissions [37]. In addition, these studies reported a subset of recording sites exhibiting selective omission responses, that is, responses to omitted but not presented sounds, suggesting an implicit predictive mechanism [37]. More recently, Fonken and colleagues replicated these findings using syllable stimuli, and showed that a subset of syllable-responsive electrodes in STG and STS also respond to their omissions [38]. Interestingly, they also found that a subset of STG electrodes showed omission but not auditory responses, indicative of distinct STG sites that selectively represent predictive processes.
Importantly, Hughes and colleagues constructed predictability by repeating streams of identical sounds [37], and Fonken and colleagues by repeating fixed sequences of three different syllables (‘La/Ba/Ga’) [38]. This implies that omission responses are not due to a release from sensory adaptation that is induced by repeated presentation of the same stimulus, but rather by a prediction built over groups of sounds. Nevertheless, Fonken and colleagues could not decode the identity of omitted sounds from HFA, suggesting that omission responses in temporal regions might be related to temporal predictions, and not to identity-specific predictions. This finding challenges evidence from past studies relying on scalp EEG [39] or fMRI [40], which showed that frequency-specific information can be decoded from omitted sounds from ERPs or hemodynamic responses in temporal brain areas. Future studies should investigate which features of omitted stimuli are mapped onto omission responses, and at which level of sensory information processing.
A variation of the classic deviance detection paradigm used for studying auditory predictions involves presenting deviant sounds at temporally predictable or unpredictable latencies [27, 41]. In one of the first of these studies, Dürschmid and colleagues examined how ERPs and HFA are modulated by deviance and predictability [27, 41]. They provided compelling evidence for a hierarchy of auditory predictions, with temporal areas being sensitive to whether a sound is a standard or deviant (i.e. low-level prediction), but agnostic to larger scale statistics (whether a deviant sound is presented in a temporally predictable way; figure 2(b)). Interestingly, frontal areas, mainly in the lateral region, showed additional HFA sensitivity to larger scale predictions [27], supporting a hierarchy of sensory predictions. In a subsequent study, they examined the role of frontal cortex in generating prediction error signals [41]. This study reported a decrease in HFA amplitude in frontal cortex before the onset of an expected compared to unexpected deviant sound, which was sensitive to the likelihood of deviance occurrence [41]. One limitation of this paradigm is that the predictability manipulation in the deviant sounds was binary, that is, their occurrence could be predicted with 100% certainty or be relatively unpredictable, while still obeying the overall statistics of the task. This means that although the precise moment of deviant sound occurrence is not known, deviant sounds still occur in 20% of the trials across the task.
More recent studies have tested probabilistic predictions, in terms of stimulus timing and identity [42]. The authors of [42] used a probabilistic paradigm inducing associations between pictures and spoken syllables, while manipulating the temporal precision of predictions and the identity of target stimuli. They found that ERPs in STG were sensitive to interactions between temporal and identity-based predictions at early latencies. Interestingly, responses in rostral middle frontal gyrus were modulated by temporal predictions (i.e. when an expected sound will occur) at early and late latencies, whereas responses in inferior frontal gyrus were modulated by identity-based predictions (i.e. which sound will occur) only at late latencies.
2.4. Regularities and predictions using naturalistic paradigms
Although most of the aforementioned studies employed non-naturalistic stimuli such as pure tones, iEEG correlates of predictive processes have also been studied using more ecologically valid paradigms. These paradigms include the learning of new grammar rules [43], predictive processes facilitating speech comprehension [28, 44], or processing of semantic information [45].
One study created a grammar containing transition rules among pseudo-words, and tested perception of sequences that were consistent with rules from this grammar, compared to rule violations [43]. HFA within primary auditory cortex (i.e. Heschl’s gyrus) was sensitive to the artificial grammar rules, as indexed by higher amplitude and inter-trial coherence for violations of established rules. Importantly, neural oscillations in primary auditory areas also showed low-frequency (3–5 Hz) phase to HFA coupling, paralleling findings in monkeys, suggesting a cross-species signature for rule learning.
In the case of speech, predictive processes have been assumed to be at play for filling-in phenomena, which involve subconsciously restoring missing parts of incoming sensory stimuli. Leonard and colleagues presented patients with sentences containing some words where one phoneme was replaced by noise, inducing a perceptual illusion, so that participants would perceive one word in some trials and another in other trials, despite identical acoustic features [28]. They showed that modulations of HFA in STG reflected which of the two words participants would perceive (figure 2(c)). Indeed, HFA responses to noise stimuli resembled HFA responses to the actual word that participants reported hearing. Similarly, investigators have used speech (here, spoken words embedded in noise) to study how experience affects neural responses to ambiguous acoustical stimuli [44]. Patients were presented with degraded speech twice, before and after being presented with a non-degraded sentence in its original form. Having heard the original spoken words facilitates comprehension, which is indexed by a perceptual enhancement of the degraded sound during its second presentation. This robust perceptual enhancement was linked to increased HFA within STG and greater similarity to the actual sentence, due to a shift in spectrotemporal tuning within STG.
Apart from sensory cortices, predictive traces during perception of naturalistic stimuli have also been found in the hippocampus. In a recent sentence processing study, Jafarpour and colleagues showed a pre-activation hippocampal pattern in anticipation of a subsequent word that would complete a sentence [45]. HFA was modulated by the predictability of the subsequent word, such that the hippocampus only showed increased activity in cases where the last word of a sentence could be easily predicted at a semantic level.
Taken together, these results suggest that predictive processes exist in the auditory modality, as shown using both artificial (e.g. relying on repetitions of short auditory stimuli) and naturalistic (speech) paradigms, and can facilitate perception and behavior. At an anatomical level, predictive circuits extend beyond primary and non-primary auditory regions to include PFC and hippocampus.
2.5. Conclusions
In summary, iEEG recordings have been used in the field of auditory regularity detection and predictions to: (a) confirm the location of primary sources underlying detection of deviant events; (b) expand the network of regions implicated in deviance detection; (c) provide a mechanistic understanding of oscillatory activity and cross-regional communication within this network; and (d) increase sensitivity on the spatial extent and existence of auditory responses. These insights challenge existing theories of regularity detection and lead to new hypotheses on this fundamental cognitive ability.
Despite progress in understanding the regularity detection network, several open questions remain. First, more evidence is needed to elucidate the role of distinct frequency sub-bands in supporting sensory predictions. A prevalent view in the field of predictive processing is that feedforward connections may be supported by HFA, mostly prevalent in superficial layers of the cortex, and feedback by low-frequency activity originating from deeper layers [46]. Future studies could investigate the direction of information flow within the auditory predictive network to provide a detailed characterization of the frequency bands and cortical layers that support the generation of predictions and prediction errors.
Second, despite ample evidence expanding the network of sensory predictions beyond two nodes, the specific contributions of each node to an extended network remain unclear. In the future, iEEG recordings may investigate which parts of this extended network operate in an ‘automatic’ way, irrespective of attention or consciousness, and which parts depend on our conscious perception of environmental regularities. Third, future iEEG studies could move towards paradigms that directly relate to real-life applications to examine how, for example, sensory predictions facilitate behavior, or support speech comprehension and communication.
3. Working and episodic memory
Our ability to think flexibly and update our autobiographies as we amass experiences from childhood through adulthood depend on memory. Episodic memory refers to the conscious formation and retrieval of memories of specific past events [47]. Working memory provides the neurocognitive horsepower that allows us to maintain and manipulate information relevant to current goals [48]. It is critical to higher-level cognitive functions, including the formation and retrieval of episodic memories. Working memory is limited in capacity to the ‘magical number 7’ for maintenance of single items (e.g. digits in a phone number) [49] or as few as ~4 items considering the practical complexities of information being maintained and manipulated in everyday life [50]. Although episodic memory is limitless in capacity [51], the limits of working memory constrain the amount of information that can be encoded into or retrieved from long-term memory stores at one time [52]. Neural processes of working memory and episodic memory encoding and retrieval may thus be inextricably linked.
In this section, we review the contributions of intracranial recordings to understanding how working and episodic memory processes are implemented in the human brain. Studies leveraging the spatiotemporal and single-trial precision of iEEG have posed the following overarching questions:
How are brain regions such as the hippocampus, known to be critical to episodic memory, involved in working memory?
What are the neural mechanisms supporting information encoding into memory?
What are the roles of different frequencies in different mnemonic processes?
3.1. Working memory
Many iEEG studies of working memory have tested the accumulation of representations in mnemonic stores by tracking time-resolved neural responses to visual stimuli (e.g. letters, faces) presented in sequence. Howard and colleagues discovered that broadband gamma activity (typically measured between 30 and 100 Hz, partially overlapping with HFA), tracked working memory load, increasing in power across lateral prefrontal and temporal cortices with the presentation of each successive letter in a sequence [53]. This effect was replicated and further localized to brain regions associated with the rehearsal of verbal information, including lateral pre-frontal, temporal, pre- and post-central cortices, and the hippocampus [54] (figure 3(a)). van Vugt and colleagues found that broadband gamma power in lateral PFC and hippocampus tracked working memory load for both letters and faces, revealing direct evidence that activity within these regions tracked load regardless of the type of visual stimulus [55]. These findings also show that working memory recruits medial temporal lobe (MTL, including the hippocampus), a brain region traditionally associated with episodic memory [56].
Figure 3.
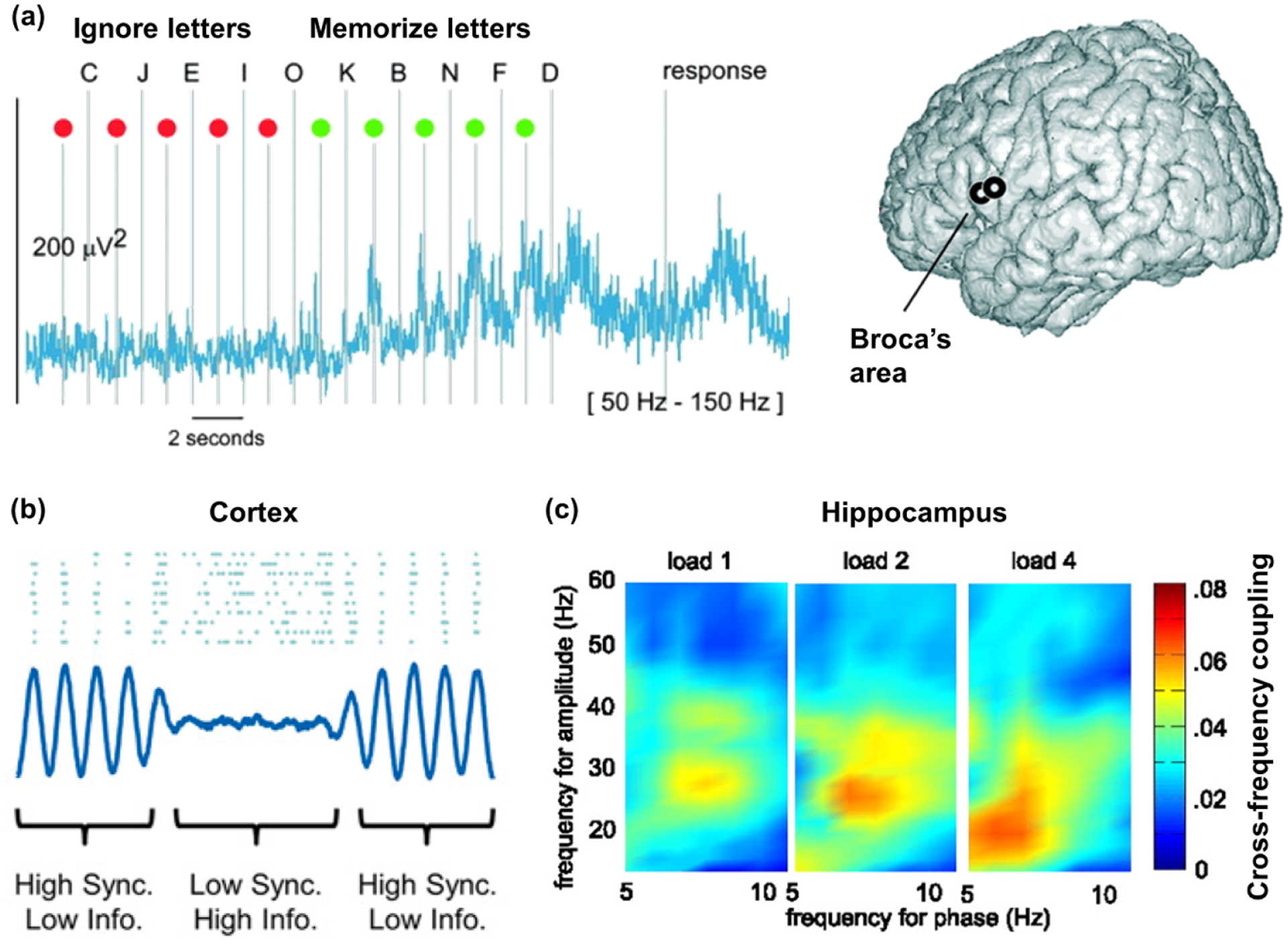
Working memory. (a) Broadband gamma activity (here, 50–150 Hz) increases with working memory load at recording sites in Broca’s area, a sub-region of PFC involved in speech production. Gamma increases steadily across the sequence of letters to memorize (green), with rapid increases at the onset of the target letters, and again with verbal response. Adapted from [54]. (b) Decreased oscillatory activity (an index of synchronization; blue line) within the cortex allows individual neurons (blue dots) to fire more freely and create a more flexible neural code for encoding information. Adapted from [57]. (c) Theta-gamma cross-frequency coupling increases with working memory load in the hippocampus. Adapted from [58].
Studies of hippocampal ERPs support the role of MTL in working memory. Axmacher and colleagues observed that negative potentials in MTL became incrementally more negative with the presentation of each letter in a sequence [59, 60]. Importantly, this ERP pattern was observed concurrent with increased broadband gamma power, and consistent with fMRI data showing increased BOLD responses during a working memory task in an independent sample, demonstrating MTL recruitment. Negative polarity hippocampal ERPs have also been shown to predict behavioral response times (RT) and accuracy [61, 62]. These findings link MTL directly to behavioral outputs, solidifying its recruitment during working memory when probed with the spatiotemporal precision of iEEG.
To further examine the relationship between broadband spectral activity and BOLD responses in working memory, Khursheed and colleagues obtained both ECoG and fMRI data from the same neurosurgical patients [63]. They found that broadband gamma and HFA (30–200 Hz) correlated with BOLD responses in lateral PFC and posterior parietal cortex (PPC) during the post-stimulus delay phase. In a task designed not to tax working memory capacity, Noy and colleagues found that the encoding and maintenance of face stimuli were associated with transient HFA responses in task-relevant visual and motor preparatory cortices (e.g. precentral gyrus) [64]. Kambara and colleagues localized HFA responses during the maintenance of four- versus two-letter sequences to the precentral gyrus [65], pointing to a role for this region in working memory. The subsequent matching of target letters to maintained sequences recruited PFC and PPC, thus showing that maintenance in precentral gyrus precedes control in PFC and PPC.
In the same ECoG-fMRI study, Khursheed and colleagues found that activity in the theta band (4–8 Hz) was inversely correlated with BOLD responses [63]. However, mounting evidence from intracranial research indicates that theta oscillations coordinate working memory representations. Indeed, neural oscillations provide optimal windows for excitability [66], and theta oscillations support neuronal computations underlying processes of information binding in mammalian MTL [67]. Raghavachari and colleagues discovered that theta activity at distributed temporal, parietal, and occipital sites tracked the temporal evolution of a working memory trial, with increased power from the onset of the first stimulus through much of the delay phase and then decreased power immediately prior to the response [68, 69]. This pattern of decreased theta evidences anticipation of a behavioral output, reflecting predictive behavioral control processes.
Two later studies revealed an inverse relationship between PFC theta power and working memory load. Meltzer and colleagues observed increased gamma concomitant with decreased theta-alpha (5–13 Hz) power during the post-stimulus delay phase throughout lateral cortical sites [70]. The difference between gamma and theta-alpha power was magnified following presentation of four items (digits) compared to one item, linking widespread theta-alpha desynchronization to load. Brzezicka and colleagues further showed that theta power in lateral PFC decreased incrementally with the successive presentation of each picture in a sequence, whereas the opposite was true for the hippocampus and anterior cingulate cortex [71]. These dual observations support a ‘synchronization-desynchronization framework’ [72]. According to this framework, these seemingly contradictory observations may reflect a division of labor between cortical sites (including PFC), which encode incoming information, and the hippocampus, which binds information to create mnemonic representations. Information encoding is most effective when cortical oscillations are not overly synchronized, that is, under conditions of low-frequency desynchronization (figure 3(b)). In contrast, maintenance of multiple pieces of information should be maximal when theta oscillations are synchronized within the hippocampus.
Four studies used sEEG to target the human hippocampus (see figure 1(d)) and investigate the role of cross-frequency theta-gamma coupling as a mechanism for maintaining information in memory stores. Axmacher and colleagues observed that maintenance of multiple items (faces) elicited theta-gamma coupling, which increased with working memory load [58] (figure 3(c)) and predicted individual working memory capacity [73, 74]. By manipulating the type of information being represented in working memory, holding load constant, our group further showed that cross-frequency coupling supported simultaneous, bidirectional interactions between MTL and PFC [75]. Without any differences in power, we found that MTL theta oscillations directed broadband gamma activity in PFC during the processing of spatial and temporal contexts, suggesting transfer of mnemonic representations from MTL. In contrast, PFC theta oscillations directed broadband gamma activity in MTL regardless of information type, reflecting control. Other studies have shown that patterns of cross-frequency coupling vary across the cortex in tasks including but not limited to working memory, illuminating the functional significance of coordination across temporal scales in distributed brain regions [76–78].
Finally, three studies tested directly whether neural oscillations coordinate activity between different brain regions during working memory. Our group tracked the evolution of functional connectivity between PFC and MTL and PPC during working memory for different types of information. We observed that maintenance of pairs of shapes was linked to unidirectional connectivity in the theta band from PPC to PFC [79] and PFC to MTL [75], pointing to a large-scale theta circuit from PPC to PFC to MTL. Critically, presentation of the test cue to retrieve specific representations from working memory stores changed this circuit from unidirectional to bidirectional. In a task of working memory for faces, Zhang and colleagues found that the strength of MTL-PFC connectivity likewise shifted with task demands in the theta band, but not in the alpha or beta band [80]. Together, these findings implicate theta mechanisms for information transmission and global network control over mnemonic representations.
3.2. Memory encoding
Many iEEG studies of episodic memory encoding have employed the subsequent memory approach, which has been widely-utilized to identify functional neural signatures of memory success [81]. By this approach, neural activity is analyzed in encoding trials as a function of whether trials are later remembered or forgotten at a subsequent memory test. Burke and colleagues tracked broadband gamma power to show the time-course of encoding across brain regions previously identified using fMRI [82]. Within 1.5 s of the presentation of each stimulus (word) that was later recalled (figure 4(a)), power first increased in the ventral visual pathway and MTL, and then rapidly across left-lateralized cortical sites associated with language, namely PFC, PPC, and temporal cortex. These spatiotemporally distinct networks suggest feedforward sensory processes are followed by PFC-related feedback control processes with sub-second precision. More recently, Kucewicz and colleagues isolated the temporal specificity of these gamma responses to the word encoding interval [83, 84]. In a study of image recognition, Johnson and colleagues found that the flow of broadband gamma and HFA between lateral PFC and precentral gyrus dictated encoding success in children and adolescents [85], corroborating feedforward followed by PFC-related feedback processes. This study also demonstrates the potential of iEEG to investigate neurodevelopment [86].
Figure 4.
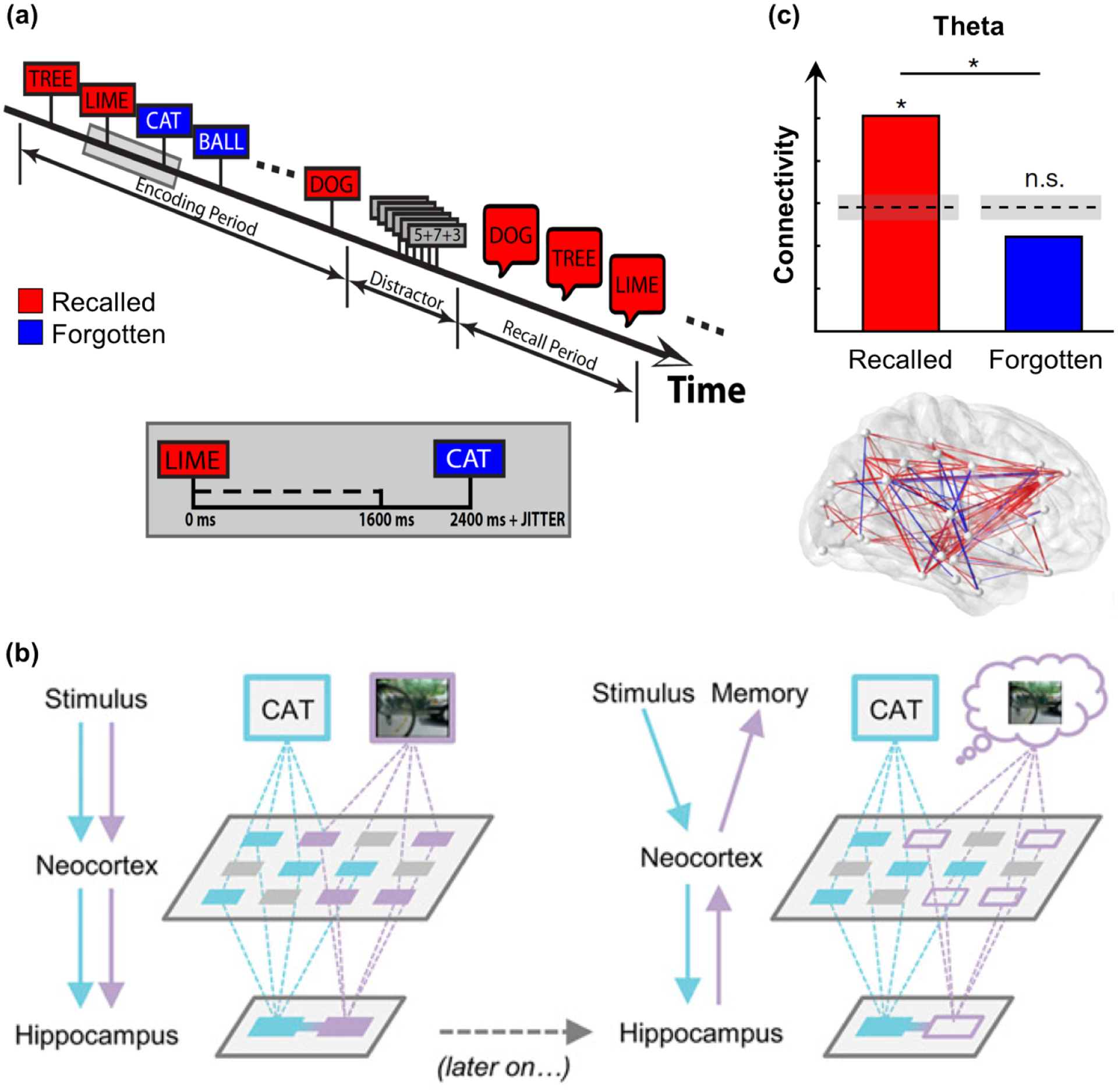
Episodic memory encoding and reinstatement. (a) In the word list recall paradigm, participants study lists of words at encoding in preparation for a free recall test. The encoding and recall blocks are separated by a distraction task (simple arithmetic) to prevent rehearsal. Inset: timeline of a single trial for a subsequently recalled word. Adapted from [87]. (b) At encoding (left), incoming stimuli (associations) are independently processed by relevant sensory regions of the cortex and then passed on to the hippocampus where they are bound together. At retrieval (right), a partial cue reactivates the hippocampal associative link, which in turn reinstates cortical patterns coding for the memory representation. Adapted from [57]. (c) Theta connectivity is increased during the encoding of words that are subsequently recalled compared to forgotten, shown in the paradigm in (a). The bar plots (top) correspond to all possible connections between pairs of electrodes (bottom). Red, recalled > forgotten; blue, recalled < forgotten; *, p < 0.01. Adapted from [88].
Several studies have reported that broadband gamma increases are observed concurrent with decreased power in the theta band during word encoding, with the most pronounced negative theta subsequent memory effects (i.e. remembered < forgotten) in frontotemporal regions [87, 89–94]. Importantly, inverse relationships between higher- and lower-frequency activities—that is, low-frequency desynchronization—during encoding are not limited to memory for words. In a study of image recognition, Matsumoto and colleagues observed that increased broadband gamma activity in temporal cortex was accompanied by desynchronized activity at lower frequencies during successful encoding [95]. In a study of word-pair associations, Sheehan and colleagues found that theta power in temporal cortex correlated negatively with subsequent performance across individuals [96]. These findings provide converging evidence for cortical signatures of information encoding, as indexed by increased broadband gamma and HFA, and low-frequency desynchronization.
Using both words and images, Fellner and colleagues recently investigated whether cortical low-frequency desynchronization might be explained by broadband spectral tilt—that is, a shift in the power spectrum favoring higher over lower frequencies during successful encoding [97] (for review see [98]). They found both increased broadband gamma and decreased theta power during the encoding of stimuli that were subsequently remembered compared to forgotten, regardless of stimulus type. However, these band-limited patterns were observed in different brain regions, with visual gamma increases preceding frontotemporal theta decreases, suggesting an explanation of different oscillatory functions. Further, Vaz and colleagues observed that cross-frequency coupling between theta oscillations and HFA activity within lateral PFC was likewise decreased during the encoding of remembered compared to forgotten words [99]. These findings corroborate dissociable oscillatory correlates of memory encoding.
According to the synchronization-desynchronization framework [72], these cortical negative theta subsequent memory effects should be accompanied by positive effects reflecting synchronized activity within the hippocampus. Indeed, positive theta subsequent memory effects (i.e. remembered > forgotten) have been observed within the hippocampus, known to be critical to episodic memory [56]. In two studies using sEEG to measure hippocampal activity during word list encoding (see figures 1(d), 4(a)), 2–5 Hz ‘slow theta’ power was found to correlate positively with broadband gamma activity and predict subsequent recall [100, 101]. Lega and colleagues further observed that the strength of cross-frequency coupling between these slow theta oscillations and gamma activity increased in the hippocampus during the encoding of recalled compared to forgotten words [102]. As in studies of working memory (see figure 3), dual observations of decreased cortical activity and increased hippocampal activity are consistent with a division of labor between regions. This division of labor is observed during the encoding and binding of multiple pieces of information regardless of the retention interval, thus linking working memory to encoding with the spatiotemporal precision of iEEG.
A requirement for information processing between cortical and hippocampal regions is evidence of information transfer between regions. One recent study tested directly whether decreased cortical activity and increased hippocampal activity were coordinated during the encoding of episodic memories. During the encoding of associations (image-word, sound-word), Griffiths and colleagues observed that decreases in low-frequency (8–20 Hz) power in lateral temporal cortex reliably preceded increases in hippocampal gamma power [57]. The authors proposed that information was first encoded in temporal cortex and then passed to the hippocampus for binding into associations (figure 4(b)), per the synchronization-desynchronization framework [72]. Another study showed that theta connectivity between the hippocampus and entorhinal cortex (within MTL), the so-called gateway to the hippocampus, increased immediately preceding increases in hippocampal gamma [94], suggesting that this proposal is likewise supported by coordination in the theta band.
Several other studies have investigated the neural mechanisms of inter-regional coordination during encoding (i.e. functional connectivity). Young and colleagues found that widespread regions, including MTL and frontal, parietal, temporal, and occipital cortices were connected in the theta band during episodic memory encoding but not in a non-episodic control task [103]. Burke and colleagues observed that global theta connectivity between wide-spread regions was maximal during the first second of the presentation of words that were subsequently recalled [87]. Successful word encoding was further linked to global connectivity in the theta band concomitant with decreased connectivity in the gamma band [88] (figure 4(c)). This double dissociation revealed theta hubs in PFC, MTL, and temporal cortex. Theta oscillations also support short-range functional connectivity between MTL sub-regions [94, 104], as well as long-range connectivity between PFC and MTL that is not limited to word encoding [105]. Together, these findings show that theta oscillations coordinate neural networks during successful encoding, implicating theta mechanisms for information transfer between brain regions in the service of memory.
Finally, Sweeney-Reed and colleagues capitalized on a rare opportunity to record directly from the thalamus, a subcortical structure that plays an important role in cortico-hippocampal networks subserving memory formation. They observed increased theta power in the dorsomedial thalamic nuclei during the pre-stimulus epoch prior to the presentation of images that were subsequently remembered compared to forgotten [106], implicating preparatory membrane excitability in the thalamus. During image presentation, they observed that theta phase alignment in the anterior thalamic nuclei predicted encoding success [107]. Both of these effects are comparable to those reported in the hippocampus by other groups [108, 109]. On a network level, they found that frontal theta oscillations modulated both frontal-thalamic theta connectivity and narrowband thalamic gamma (40–50 Hz) activity during successful encoding [110], consistent with control from PFC. These findings further implicate theta mechanisms in global network coordination underpinning successful encoding, supporting both information transmission and control processes.
3.3. Memory retrieval
The principle of encoding specificity states that retrieval is most effective when retrieval conditions are similar to encoding conditions at the time of memory formation [111]. Evidence for context-dependent encoding specificity may manifest as the subsequent reinstatement of iEEG activity patterns observed during encoding (i.e. remembered vs. forgotten). Using the same word recall paradigm shown in figure 4(a), Kragel and colleagues found that engagement of core memory regions, including MTL, temporal cortex, PFC, and PPC, predicted both encoding and retrieval success as indexed by increased HFA and low-frequency (3–10 Hz) desynchronization [93]. Similar patterns of HFA suggest that successful recall begins in the hippocampus and then spreads to temporal cortex and PFC [112]. Further scrutiny of correctly recalled words compared to both false recall of new words and periods of no recall revealed that the hippocampus was selectively involved in recall accuracy [113]. HFA in the hippocampus has also been shown to predict recognition performance across individuals [114].
Like encoding, Staresina and colleagues observed hippocampal reinstatement of word-color associations, as reflected in both increased broadband gamma activity and decreased alpha (8–12 Hz) activity [115]. The authors proposed that pattern completion (i.e. putting the pieces of prior experience together) was dually supported by dissociable gamma mechanisms for selection of target-relevant neurons and alpha mechanisms for the reinstatement of information content. Consistent with dissociable oscillatory correlates at both encoding and retrieval, Vaz and colleagues found that, like word encoding, word retrieval was linked to decreased cross-frequency coupling between theta oscillations and gamma activity in temporal cortex [99].
Several studies have shown a reversal in the direction of cortico-hippocampal connectivity patterns between encoding and retrieval. In the same study of associations shown in figure 4(b), Griffiths and colleagues observed that increases in hippocampal gamma power reliably preceded decreases in low-frequency (8–20 Hz) power in temporal cortex, opposite of the pattern observed at encoding [57]. Here, the authors proposed that retrieval was associated with hippocampal pattern completion inducing reinstatement of the remembered information in temporal cortex. Further support for this proposal comes from a study of item-context associations, in which hippocampal delta-theta (1–8 Hz) power was greater for retrieved associations compared to the same items in new contexts, and preceded broadband (1–100 Hz) reinstatement in temporal cortex [116]. Likewise, delta (1–3 Hz) oscillations shifted such that visual encoding was associated with connectivity from cortical to hippocampal MTL sites, and retrieval with connectivity from hippocampal to cortical MTL sites [117].
During the retrieval of items encoded during virtual navigation, Herweg and colleagues observed that items were recalled according to how they were clustered at encoding [118]. Spatial clustering was associated with increased hippocampal theta power preceding verbal recall, as well as hippocampal theta modulation of HFA in MTL cortex. A recent study further showed that hippocampal theta coordinated memory-guided eye movements during the retrieval of spatial locations [119]. These findings implicate hippocampal reinstatement of spatial context, guiding behavior, and suggest transfer of spatial information from the hippocampus. Theta oscillations have also been shown to support short-range functional connectivity between MTL sub-regions at retrieval, as in encoding [94], as well as long-range connectivity between MTL and PFC and PPC [120]. Outside MTL, Bauch and colleagues observed increased thalamic theta power and connectivity to nucleus accumbens during the successful retrieval of old images compared to the correct rejection of new images [121]. These findings corroborate theta mechanisms for global network coordination during both encoding and retrieval (see figure 4(c)), likely reflecting information transfer and control processes.
In everyday life, however, episodic retrieval often requires differentiating between memories that overlap in some way (i.e. pattern separation). Here, reinstatement may be accompanied by neural signatures of disambiguation between mnemonic representations and new, similar representations presented at retrieval. Lohnas and colleagues analyzed multivariate patterns of broadband gamma activity during the continuous recognition of repeated and similar images [122]. Reinstatement was observed in occipitotemporal cortex during both successful recognition of repeated images and viewing of images that were similar but not identical to those presented previously. In contrast, the hippocampus differentiated between repeated and similar images, supporting its role in pattern separation during encoding and retrieval. Stevenson and colleagues found that broadband gamma power in the hippocampal CA1 region (see figure 1(d)), but not other MTL sub-regions, predicted the precision of mnemonic representations which overlapped in space [123]. Zheng and colleagues showed that theta coordination between the hippocampus and amygdala, involved in emotional processing, predicted the accuracy of representations which overlapped in emotional content [124]. El-Kalliny and colleagues found that patterns of low-frequency (3–12 Hz) activity in temporal cortex differentiated between memories that overlapped in time and predicted performance, suggesting that retrieval of specific memories is also facilitated by pattern separation in temporal cortex [125]. Together, these findings demonstrate signatures of both pattern completion and separation in the hippocampus and temporal cortex, subserving successful episodic memory retrieval.
Last, Axmacher and colleagues discovered that MTL ripples are involved in memory retrieval [126]. Ripples are transient bursts of narrowband oscillations, typically measured between 80 and 140 Hz, which reflect population-level neuronal firing and are widely observed in animal models during memory tasks. They found that MTL ripple rate correlated with participants’ recognition of images. Two recent studies further revealed a role for hippocampal ripples in both encoding and retrieval processes. Norman and colleagues observed that the ripple rate was increased during the encoding of images (faces, places) that were subsequently recalled compared to forgotten and reinstated during the seconds immediately preceding recall [1]. HFA in higher-order ventral visual regions also coupled to hippocampal ripples during successful retrieval, consistent with the dissociable processing of faces and places. Vaz and colleagues observed comparable MTL ripple reinstatement effects during the encoding and retrieval of word-pair associations, as well as coupling between MTL and temporal cortex during successful retrieval [127]. These findings show that MTL ripples support encoding and retrieval and may coordinate the reinstatement of information processed in content-specific cortical sites.
3.4. Conclusions
Findings from iEEG studies of working and episodic memory converge on four major insights: (a) working memory recruits MTL and not only cortical structures; (b) cortical gamma/low-frequency desynchronization supports information encoding and reinstatement; (c) MTL theta activity and theta-gamma cross-frequency coupling support information maintenance and binding into episodes; and (d) inter-regional theta connectivity provides the functional infrastructure for information transfer and control. These insights link working memory to processes of episodic encoding and retrieval, including but not limited to information encoding, maintenance, and transfer, utilizing the precision of iEEG.
However, fundamental questions about the neural mechanisms of mnemonic processes remain. For instance, in seeming conflict with noninvasive EEG [98], iEEG studies have shown that decreased power in the theta band and other low frequencies is associated with increased working memory load [70, 71] and successful compared to unsuccessful encoding [87, 89–95]. By demonstrating that low-frequency desynchronization is not a byproduct of increased broadband power, but a distinct process during memory formation [97], iEEG evidence argues against the adage ‘more is better’. Made possible with the spatiotemporal precision of iEEG, evidence instead suggests a division of labor between brain regions; cortical regions involved in information encoding exhibit low-frequency desynchronization while the hippocampus and other medial regions involved in binding information into episodes exhibit the opposite pattern (see figure 3(b)) [72]. Indeed, there is evidence suggesting that increased theta over PFC in the scalp EEG topography has a medial source [71]. Nonetheless, it is not clear what mechanism is reflected by desynchronization, per se, illuminating the need for future research that provides mechanistic explanations of the neural basis of human memory.
4. Internal cognition
In line with the zeitgeist of cognitive and systems neuroscience, iEEG studies of human cognition have historically focused on sensorimotor functions [128, 129]. These are externally oriented processes that recruit primary cortical areas responsible for perception and action, including auditory regularity detection [24–26] as described in section 2, visuospatial processes [130, 131], and motor processes [11, 132]. According to the principal gradient of large-scale network organization [133], these unimodal regions fall on one end of the spectrum. On the opposite end of the spectrum lies the transmodal regions, which include the default mode network (DMN) in humans. The DMN is a network of regions that have traditionally been shown to activate during rest and deactivate during externally oriented cognitive tasks [134, 135]. The principal gradient provides a spatial framework that accounts for the DMN’s role in internally oriented processes. In particular, the DMN’s position in this hierarchical organization of the cortex, far away from the unimodal regions, allows it to process transmodal information, unconstrained by sensory inputs, which explains its involvement in internal cognition.
The discovery of the DMN has fundamentally shifted how we conceptualize the functional organization of the brain. Indeed, the observation that the brain is active at rest led to a serendipitous outcome of increased interest in internally oriented cognition. In contrast to auditory perception (section 2) or certain types of memory functions (section 3) that rely on externally presented information, internal cognition does not require external inputs as it involves cognitive processes that are oriented internally towards our own thoughts. This includes processes commonly known as mind wandering, theory of mind, and autobiographical memory retrieval, among others. In particular, mind wandering reflects periods of time when attention is not focused on the external task, whereas theory of mind involves attributing mental states to ourselves and others. Autobiographical memory retrieval is related to episodic memory retrieval (as described in section 3.3) in that it refers to memory of past events but is distinct in that the memory involves the self. Given that internal cognition is an inherently covert process that often occurs in the absence of external inputs, it lacks robust overt behavioral markers, necessitating objective measures that can reliably and directly capture this covert phenomenon. Neuroimaging methods provide such objective measures that effectively characterize the neural activity and connectivity patterns associated with covert cognitive processes. Here, we focus on studies that employ iEEG to further delineate the neural underpinnings of internal cognition.
The aforementioned advantages of iEEG (i.e. high spatiotemporal precision and signal-to-noise ratio) apply to the study of internal cognition. In particular, internally oriented cognitive functions are considered to be intrinsically dynamic and fast acting, rendering EEG an ideal tool for capturing these transient processes. Importantly, iEEG offers access to brain regions (e.g. DMN) relevant to internal cognition that are not easily accessible by scalp EEG or MEG [136]. Taken together, iEEG offers both the spatial and temporal resolution ideal for understanding the structures and mechanisms underlying internal cognition.
Broadly, this section will highlight iEEG studies involving the most commonly studied types of internal cognition: rest and autobiographical memory retrieval. Leveraging the advantages of this methodology, these studies used iEEG to investigate questions about internal cognition that noninvasive neuroimaging methods are not equipped to address. These overarching questions include:
Does the DMN play a singular role in internal cognition, or is there spatial heterogeneity and temporal variation in its involvement in internal cognition?
What are the electrophysiological mechanisms within and across the involved neural networks underlying internal cognition?
4.1. Rest
Functional neuroimaging data revealed numerous networks that are spontaneously engaged during resting state. As these are periods of time when participants were asked to simply do nothing, increases in the fMRI BOLD signal during rest are thought to reflect an intrinsic property of the brain [134]. The observation that neural networks are recruited when participants are passively staring at a screen and not actively performing a task piqued interest in the notion that the brain is not idle in the absence of an external task. These studies set the foundation for internal cognition research in cognitive neuroscience [137, 138]. Accordingly, before delving into actively engaged internally oriented cognitive processes, we review studies that use iEEG to address the following questions about the resting state:
How does the electrophysiological response correspond with the BOLD response in networks recruited during resting state?
What is the temporal profile of the electrophysiological response during resting state?
What are the neural mechanisms supporting resting state?
Foundational fMRI studies revealed multiple regions that are intrinsically connected during rest, collectively known as the resting state networks. These networks are comprised of regions that are anatomically distinct but functionally connected, usually indexed by temporal correlations of the BOLD signal [139], wherein their patterns of connectivity persist across cognitive states [140] and are consistently observed across individuals [141]. In line with functional connectivity findings in fMRI, Kucyi and colleagues reported evidence of strikingly similar connectivity patterns between HFA using iEEG and the BOLD signal using fMRI in the same brains [142] (figure 5(a)). Their analyses focused on connectivity within three resting state networks: the DMN, dorsal attention network (DAN), and frontoparietal control network (FPCN). Relationships between low-frequency activity and the BOLD signal are less consistent, with studies showing positive correlations within networks [142] but negative correlations in different brain regions [143]. In examining connectivity between resting state networks, a recent study found a modest negative correlation in HFA between the DMN and DAN [144], supporting early neuroimaging findings of antagonistic activity between these networks [145]. Together, these within and across network findings provide a neurophysiological basis for the connectivity patterns observed in the fMRI BOLD signal.
Figure 5.
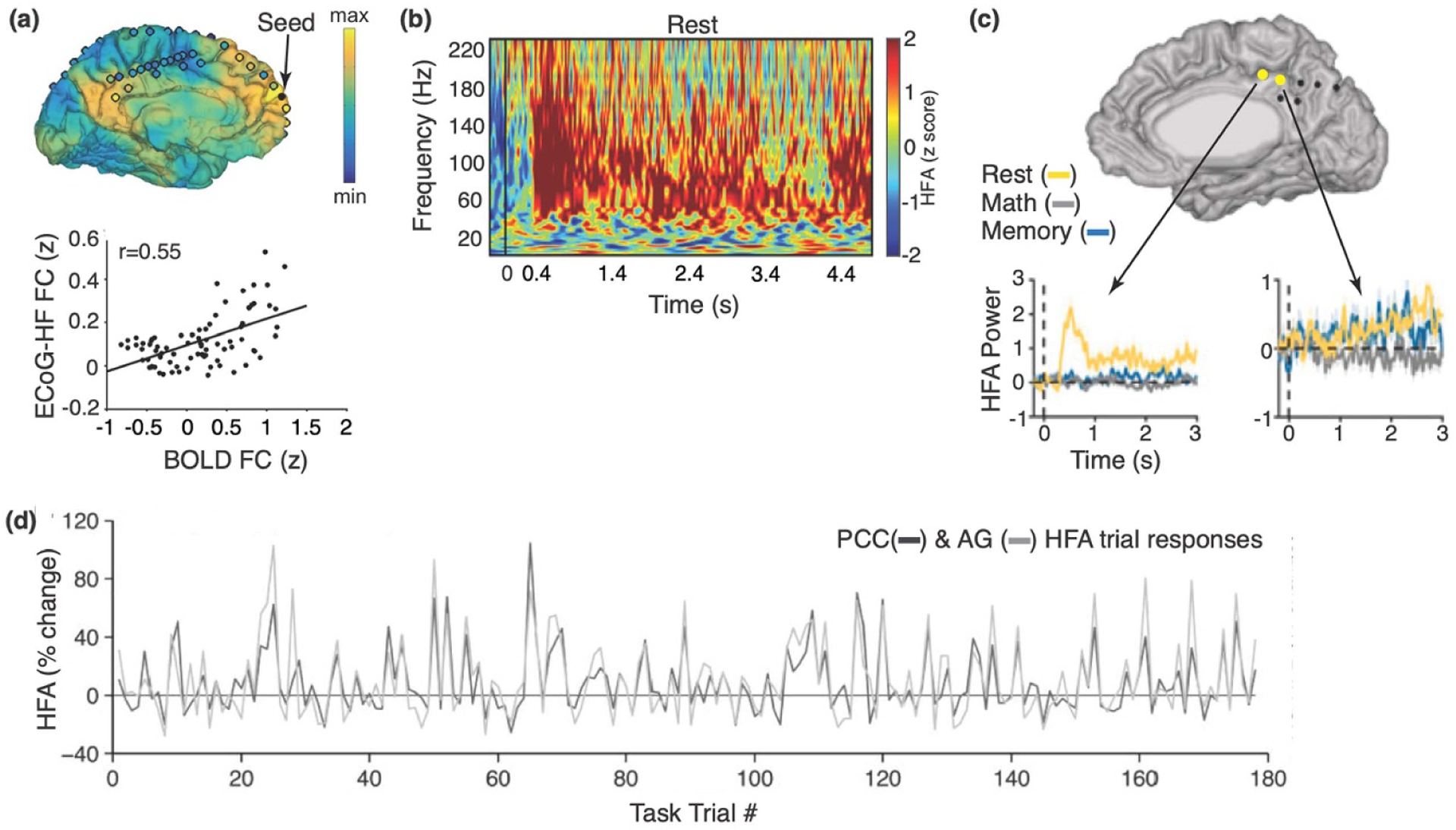
Internal attention. (a) Positive correlation between iEEG HFA and fMRI BOLD seed-based functional connectivity (FC) in one patient. For iEEG HFA, electrodes are shown as circles filled with colors representing magnitude of correlation between itself and the seed (colored in black) embedded in the DMN. For fMRI BOLD, the magnitude of correlation between the seed and rest of the brain is shown on the pial surface. Adapted from [142]. (b) Increased HFA during rest for a representative electrode in the DMN. Adapted from [146]. (c) Differential temporal profile of two neighboring electrodes in the DMN. The representative electrode on the left shows increased HFA time-locked to rest onset whereas the electrode on the right shows jittered HFA throughout the rest trial (yellow trace). Adapted from [147]. (d) Strong positive correlation in trial-by-trial HFA responses between posterior cingulate cortex (PCC; black trace) and angular gyrus (AG; grey trace), two regions within the DMN. Adapted from [148].
Given the unique role of the DMN in internal cognition, several studies specifically examined the spatial and temporal profiles of neural responses in the DMN during rest. In particular, electrodes implanted in core regions of the DMN, including medial prefrontal cortex and posterior cingulate cortex/precuneus, showed increased HFA during sustained periods of rest [149]. Dastjerdi and colleagues reported similar findings in posterior medial cortex [146], a core hub of the DMN that is structurally and functionally connected to other parts of the DMN [150]. They found increased broadband gamma and HFA in posterior medial cortex during rest regardless of its duration (i.e. 5 s and 3 min) and condition (eyes open and closed). In the 5 s condition, HFA peaked approximately 400 ms post onset of rest and was sustained throughout the trial (figure 5(b)). Furthermore, electrodes that showed shorter onset latency transitioning into rest periods also showed greater HFA.
Extending these findings, Daitch and Parvizi reported that dorsal relative to ventral posterior medial cortex was more likely to show greater HFA during rest with eyes open [147]. Given that the spatial resolution of iEEG can reach inter-electrode distances of 3–4 mm, this method enables the examination of neural patterns from sites in posterior medial cortex at large and within its subareas. To that end, this study also found variable temporal patterns across these neighboring electrode sites (figure 5(c)). Specifically, some electrodes showed a transient increase following the onset of cued rest which quickly returned to baseline. The authors interpreted this pattern as sensitivity to the transition into rest. In contrast, other electrodes showed bursts of activity throughout the trial, thought to reflect the dynamic nature of ongoing thoughts during rest. Collectively, these studies revealed the spatial and temporal heterogeneity of the DMN.
Using a similar experimental paradigm, Foster and colleagues observed that the magnitude of HFA was modulated by the phase of theta within the same electrodes in posterior medial cortex [151]. As previously mentioned, cross-frequency coupling has been proposed to facilitate communication across temporal scales [76], and play a functional role in the integration of spatiotemporal dynamics within large-scale brain networks [152]. Importantly, they found a peak in theta oscillations in posterior medial cortex, which stands in contrast to the alpha peak in the neighboring occipital cortices. This difference in oscillatory frequency between adjacent cortical regions underscores the value of using iEEG to capture specialization across structurally adjacent but functionally distinct regions. These findings suggest that theta oscillations may be a spectral signature of posterior medial cortex within the DMN during rest, coordinating local activity at different time scales.
In summary, these studies: (a) established the neurophysiological basis for the connectivity patterns observed in the BOLD fMRI signal during resting state; (b) provided evidence for variable temporal patterns that map onto spatial heterogeneity within the DMN at rest; and (c) identified cross-frequency coupling as one electrophysiological mechanism within the DMN that underlies the resting state. Given that the core hubs of the DMN consist of medial prefrontal and parietal cortices, iEEG offers unique access to these medial structures, with the temporal precision necessary to identify neural mechanisms and preferred frequencies.
Rest is commonly characterized by periods of time when participants are lying in the scanner and instructed to think about whatever comes to mind. Although this operationalization of rest has revealed the intrinsic networks that emerge in a task-free environment, it does not inform the experimenter about a participant’s focus of attention or the content of their ongoing thoughts. Notably, the absence of a task means no constraints are placed on what goes on in participants’ minds. Whether resting state is recorded with eyes open (in which participants passively view a blank screen) or eyes closed, attention is presumed to be internally focused. However, experimental constraints are not usually in place to ensure that the observed neural activity recorded during rest is necessarily capturing internal cognition. This lack of insight into a participant’s attentional focus and ongoing thoughts during rest not only characterizes the studies summarized here, but also the majority of resting state studies. To better understand the functional role of neurophysiological activity observed during resting state, future studies are encouraged to consider obtaining measures that characterize ongoing cognitive processes during the resting state. In the next section, we review iEEG findings in experimental contexts in which the internal cognitive states were task-induced and experimentally controlled to better understand the neural underpinnings of internal cognition.
4.2. Internally oriented cognitive processes
Internal cognition refers to cognitive processes that are focused on the inner milieu of an individual. With the aim to characterize the types of internally oriented thoughts, studies have revealed that the majority of our time spent in internal cognition is focused on the past or future [153]. Recent developments have begun to explore how internally oriented thoughts dynamically unfold over time [154] and contrast thoughts that arise with or without intention [155]. Notably, studies employing iEEG to examine signatures of internal cognition have primarily focused on autobiographical memory retrieval. Accordingly, this subsection reviews iEEG studies in a task-based context with emphasis on the neural regions and electrophysiological mechanisms within and across these regions underlying internal cognition as it pertains to autobiographical memory retrieval. These studies address two main questions:
What does the spatial heterogeneity within the DMN inform us about the functional specialization of this network during internal cognition?
Are there dissociable electrophysiological patterns underlying the intra- and inter-network connections with the DMN?
Consistent with noninvasive neuroimaging literature, converging evidence from iEEG studies implicates the DMN, and posterior medial cortex in particular, in internal cognition. Several studies have converged on the functional specificity of this region. In one study, electrodes in posterior medial cortex showed increased HFA during autobiographical memory retrieval compared to math processing, an externally oriented process [156]. Remarkably, the extent of increased activity during autobiographical memory retrieval predicted the magnitude of decreased activity during math processing. This finding indicates that posterior medial cortex is specifically engaged during an internally oriented process and suppressed during an externally oriented process, revealing its functional specificity. Similar to the onset of activity during rest, HFA also onsets at around 400 ms during autobiographical memory task trials [150]. The functional specificity of posterior medial cortex electrodes was substantiated by a recent study that compared two types of internally oriented processes [147]. Although some overlapping electrode sites were recruited during both autobiographical memory retrieval and rest, most sites were distinctively active during one condition and not the other. Together, these findings suggest that posterior medial cortex is broadly recruited for internal cognition, and subareas within it are differentially involved in different types of internal cognition, highlighting the detailed functional specialization of posterior medial cortex and the exquisite spatial resolution of iEEG.
Such functional specificity corresponds to the spatial heterogeneity of posterior medial cortex. In terms of broad spatial characterization, a distinction has been made between the dorsal and ventral parts of posterior medial cortex. Specifically, Dastjerdi and colleagues reported the initial observation of two electrode sites in the ventral part of posterior medial cortex that specifically showed increased HFA during autobiographical memory retrieval [146]. This was corroborated by subsequent observation with a larger sample of electrodes and patients [147]. The same study reported that the dorsal part of posterior medial cortex was active during cued rest, supporting the notion that rest is not necessarily analogous to other internal cognitive processes. Another study reported involvement of both ventral and dorsal parts of posterior medial cortex during such as autobiographical memory retrieval; however, a distinct temporal profile was observed [156]. Specifically, the ventral sites exhibited earlier HFA onsets and more sustained activity than dorsal sites, whereas dorsal sites exhibited the opposite pattern. These findings exemplify the value of iEEG in revealing functionally distinct temporal profiles amongst neighboring electrode sites that are only millimeters apart within the same anatomical boundary.
In addition to posterior medial cortex, other areas within the DMN have been implicated in internal cognition, including retrosplenial cortex and angular gyrus [148]. HFA in these three DMN regions was highly correlated at the single-trial level across all task conditions and onset simultaneously (figure 5(d)), suggesting that these areas operate in parallel in support of autobiographical memory retrieval. Although neuroimaging studies have identified these areas as different hubs of the DMN [150], these findings illustrate that they do work together within millisecond resolution during autobiographical memory retrieval. Notably, these patterns were observed only within these areas and were specific to HFA responses. In examining whether similar patterns might also be observed in other states, the authors found that the connectivity between these three areas, as indexed by slow (<1 Hz) fluctuations of HFA, were observed during task conditions and reinstated during rest and sleep. These recurring connectivity patterns underscore the importance of understanding intrinsic patterns of electrophysiological activity, and the insights they provide into the functional coordination of large-scale brain networks.
Beyond within-network communication, our group recently examined the interaction between the DMN and FPCN in support of internal attention [157]. Although the FPCN is strikingly distinct from the DMN, its subsystem A in particular has been implicated in internal cognition [158–160]. In examining the interactions between electrodes within these two networks, we found increased theta-band connectivity during internal compared to the external attention condition, during which participants were instructed to attend to the internal or external environment. Consistent with studies suggesting the selectivity of theta oscillations during internal attention processes [161], particularly within the DMN and FPCN [151, 162], this pattern was not observed in other low frequency bands. Further, the inter-network theta connectivity measure predicted attention ratings for the internal attention condition, highlighting its functional role in facilitating internally oriented cognitive processes. These findings provide electrophysiological evidence for enhanced functional coupling in the theta band between these two networks during internal attention.
In summary, iEEG studies with a focus on task-induced activity have revealed spatial and temporal heterogeneity in electrophysiological responses within the DMN, and the posterior medial cortex in particular, highlighting the functional specialization of the DMN. These studies also uncovered dissociable preferred frequency ranges for intra- and inter-network connectivity during internal cognition: coordination within the DMN nodes occurs in the high-frequency range observed at the single-trial level, whereas connections between large-scale brain networks is observed at the lower-frequency theta range. These insights are not readily obtained from noninvasive neuroimaging methods. Rather, the spatiotemporal precision of iEEG afforded these studies the capability to reveal variable temporal patterns in electrodes that are millimeters apart, and distinct frequencies and forms of inter-regional coordination within and between large-scale brain networks subserving internal cognition.
These studies are not without limitations. For example, while sparse sampling of neural activity is a general limitation of iEEG, high-level cognitive functions such as internal cognition that recruit a widespread network of regions are impacted by the restricted access to subsets of brain regions. Future studies may consider using a model-based approach to infer whole brain activity based on the restricted set of sampled electrodes in iEEG [163].
4.3. Conclusions
In this section, we synthesized studies using iEEG that revealed the neural underpinnings of internal cognition, including resting state, autobiographical memory retrieval, and internal attention. Collectively, these studies provided several insights into the neural structure and mechanisms underlying internal cognitive processes.
First, the DMN is consistently engaged during internal cognition, with evidence suggesting that various parts of the network operate in parallel in support of internally oriented cognitive processes. A series of studies using the same paradigm and similar analytical techniques also provided converging evidence for the anatomical selectivity of the DMN. In particular, the ventral part of posterior medial cortex plays a prominent role in autobiographical memory retrieval whereas the dorsal part is more engaged during rest. Second, electrodes in the DMN exhibit functionally distinct temporal profiles. Communication within and across networks appears to operate at both slow and fast timescales. Whereas most studies implicated HFA within networks, which spontaneously fluctuates at a slow scale of below 1 Hz, evidence also supports the role of theta within the DMN as well as its interaction with the FPCN. Finally, the various types of internal cognition recruit several sub-regions of the DMN and rely on different electrophysiological mechanisms, underscoring the functional specificity of the DMN. Taken together, leveraging the spatial and temporal precision of iEEG enabled these studies to reveal the electrophysiology subserving different forms of internal cognition.
5. Causality and cognition
In the previous sections, we reviewed neural signatures of auditory perception, working and episodic memory, and internal cognition, examined through the precise lens of iEEG. These insights bring us closer to understanding not just where and when such fundamental cognitive processes are implemented in the human brain, but how—a primary goal of cognitive neuroscience. Understanding the precise functional neurophysiology of human cognition is also prerequisite to successful intervention following loss of function from common insults such as traumatic brain injury or stroke. To this end, research aims to progress beyond the study of neural correlates toward uncovering causal mechanisms which give rise to cognition. In this section, we highlight insights from iEEG that permit inference of causality. We focus specifically on invasive electrical stimulation as a tool to infer causality through the temporary manipulation of spatially precise brain structures and spatiotemporally precise neural functions. Overarching questions in studies using intracranial electrical stimulation (iES) to engineer neurocognitive functions include:
Which brain structures are causally involved in specific cognitive functions?
Which frequencies are causally involved in specific cognitive functions?
Why are some cognitive behaviors disrupted and others facilitated by iES?
5.1. Causality of structure
Just as iEEG electrodes can record electrical activity from the underlying neural tissue, they can also be used to deliver spatiotemporally precise electric pulses to the same tissue (i.e. iES; figure 6(a)). Clinicians typically utilize intracranial, extra-operative stimulation at a fixed gamma frequency (50 or 60 Hz, 2–10 mA current, 200–300 μs pulse width) to perform functional mapping that transiently disrupts primary language and motor sites. This practice was pioneered by Penfield and colleagues in the 1930s [164]. By temporarily perturbing function via iES of paired electrodes and tracking changes in behavioral outputs, clinicians define the spatial extent of regions critical to language and motor functions and spare these regions from surgical resection. By ‘turning off’ a stimulated region and disrupting a specific behavior, these mapping data link brain structure with millimeter precision directly to behavioral outcomes and are thus appropriate to infer causality. iES can also be used to induce perceptual phenomena and probe network connectivity, making the iES mapping approach appropriate to address causality in research as well.
Figure 6.
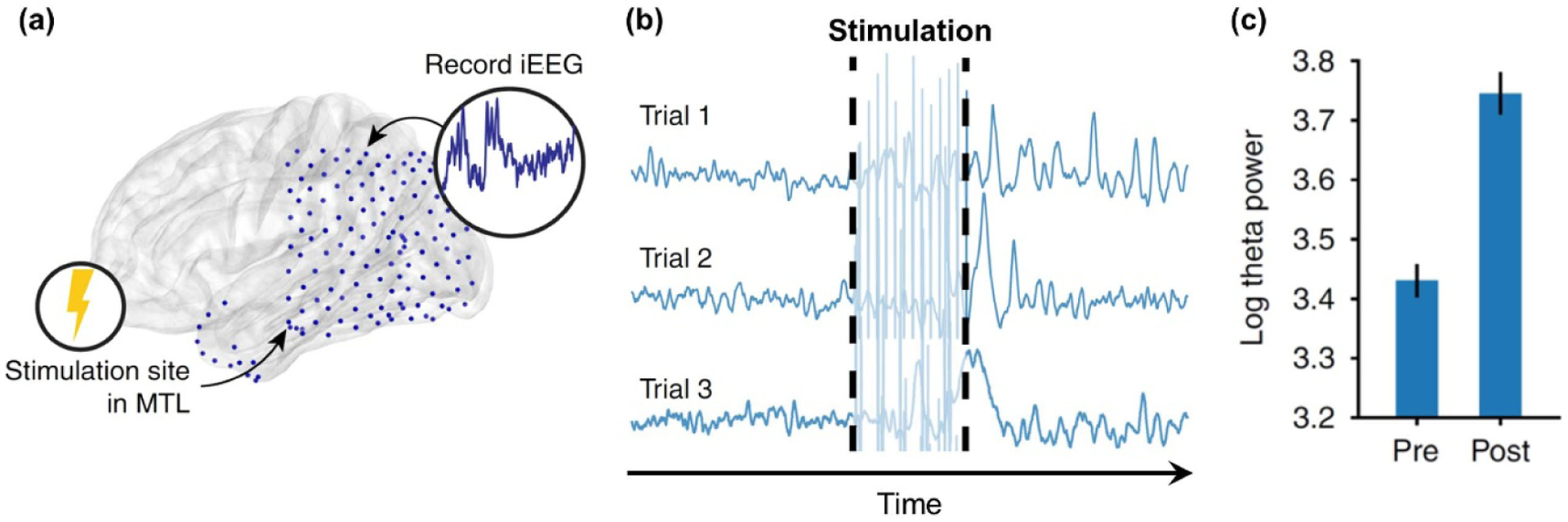
Electrical stimulation. Adapted from [165]. (a) Representative patient receiving iES to MTL. (b) Single-trial theta-filtered signals at the site stimulated in (a). Here, MTL was stimulated at the theta frequency. Note the increase in activity from pre- to post-stimulation. (c) Theta power at the stimulated site increases with theta stimulation.
In a series of disruptive iES studies, Parvizi and colleagues demonstrated that stimulation of the fusiform face area, but not sites outside this area, disrupted patients’ ability to recognize faces, linking this area directly to face perception [166, 167]. Likewise, stimulation of the parahippocampal place area was found to induce hallucinations of places [168]. By showing that perturbation of these higher-order visual regions elicits region-specific changes in behavioral outputs, these studies confirm their causal role in specific higher-order visual functions. However, whereas a shift in behavior in either direction evidences a causal link between structure and behavior, it remains an open question why iES sometimes elicits and other times disrupts behavior. Perhaps, consistent with clinical mapping, a specific cognitive behavior is elicited by ‘turning off’ a region causally involved in inhibiting that behavior. Alternatively, it is likely that more nuanced structure-function-behavior relationships exist [169]. We outline studies aiming to disentangle these relationships.
In a 1998 study, Fried and colleagues stimulated electrodes distributed across left frontal cortex of a 16-year-old patient and found that stimulation within a 2 × 2 cm region in the anterior supplementary motor area consistently elicited laughter [170]. Furthermore, the duration and intensity of laughter increased with stimulation intensity whereas other movements ceased, and speech arrest without laughter was induced by stimulation of adjacent electrodes outside this small region. Results indicated that speech and laughter are closely represented, yet distinct, in the supplementary motor area. More recently, another group showed that stimulation of the frontal operculum, which connects the voluntary motor system and limbic emotional network, induced smiling or laughter in all four patients tested [171]. These results may tap into a broader network which governs, among other things, laughter. Indeed, stimulation of the frontal operculum has been shown to elicit variable behaviors, including language-related and somatosensory effects, and widespread evoked potentials in specific non-stimulated cortical sites [172]. These findings further reveal the connectivity patterns within operculum networks that underlie various behaviors.
Relevant to memory (section 3), Aminoff and colleagues observed that stimulation of ventromedial temporal cortex, part of the ventral visual stream involved in object perception, elicited memory-related, visual hallucinations of objects that had been previously associated [173]. Indeed, studies of Penfield in the 1960s reported that intracranial stimulation of temporal cortex activated visual and auditory memory representations of past experiences [174], replicated in [175]. More recently, Kucewicz and colleagues reported that stimulation of temporal cortex enhanced verbal recall (see figure 4(a)) [176, 177]. These studies confirm causal links between temporal cortex and mnemonic representations.
Relevant to internal cognition (section 4), Fox and colleagues found that stimulation of the temporopolar cortex impacted participants’ ability to initiate self-generated thoughts [178]. This region is densely connected to orbitofrontal cortex and MTL and may be involved in integrating affect and perception. Indeed, stimulation of orbitofrontal cortex has been shown to reliably change the subjective experience of affective and somatosensory phenomena, such as eliciting experiences of sadness, anger, and aversive smells and tastes, linking affect and perception [179]. iES has also revealed a causal role for MTL, critical to episodic memory [56], in self-generated thoughts and thus links mnemonic processes to internally oriented processes (reviewed in [180]). However, stimulation of posterior medial cortex, a core hub of the DMN involved in autobiographical memory, was found not to impact autobiographical recall [181]. Although posterior medial cortex is consistently recruited during autobiographical memory, iES data suggest that this region alone may not be necessary in facilitating autobiographical memory processes known to rely on distributed brain regions.
Several other studies have suggested causal associations between different brain regions and different cognitive phenomena. In a case study, stimulation of anterior cingulate cortex elicited the expectation of an imminent challenge together with the ‘will to persevere’ and overcome it [182]. Resting-state connectivity analysis further revealed that the stimulation site was connected to a network involving fronto-insular and frontopolar regions that together may give rise to such feelings of distress and perseverance. iES data have also provided causal links between the temporo-parietal junction and out-of-body experiences [183], medial parietal sites and vertigo [184], and PFC and intrusive thoughts [185].
Finally, studies of cognitive phenomena may apply clinical mapping data to research without additional electrical stimulation. Clinical mapping is standard practice prior to surgical resection and the data are thus available for other analyses. Instead of temporarily manipulating the brain during performance of cognitive tasks, researchers may consider how distributed sites of known language or motor function are involved in task-related processes. For instance, iES data are directly applicable to auditory perception (section 2) through mapping of audio-visual comprehension [186] or language-related regions, which parallel findings from iEEG recordings [187]. Further, Ofen and colleagues recently suggested that mapping data be used in the burgeoning field of pediatric intracranial research to disentangle the involvement of language and motor sites in the development of memory [86]. That is, complex cognitive phenomena such as memory recruit multiple neural systems, some central and others that may be peripherally involved (e.g. language to memorize words, motor function to make responses). Mapping data may thus be applied broadly in intracranial research that seeks to understand the neural basis of human cognition.
5.2. Causality of function
To infer the causality of function, researchers extra-operatively stimulate paired electrodes at frequencies associated with specific brain regions and functions. Similar to the noninvasive technique of transcranial alternating current stimulation, this procedure modulates neural activity by entraining oscillations to the stimulated frequency [188]. By tracking changes in neural measures and behavioral outputs with the spatiotemporal resolution and single-trial precision of iEEG (figure 6), these data link brain function at high-resolution directly to behavior and thus are appropriate to infer causality. Like iES studies of the causality of structure, frequency-specific stimulation can facilitate or disrupt specific cognitive behaviors. However, instead of employing a unidirectional procedure purported to ‘turn off’ the stimulated region, these studies can impact neural function in both directions (e.g. by increasing activity at the stimulated frequency, which correlates positively or negatively with behavioral outcomes). In this way, the direction of effects lends further insight into how spatiotemporally precise neural activity governs cognitive performance. Outcomes have implications for both basic science and intervention.
Many of the studies published in the last several years have targeted functions associated with memory in effort to improve performance. In a study of working memory (section 3.1), Alagapan and colleagues stimulated the superior part of lateral PFC at individuals’ peak task-evoked low-frequency (4–12 Hz, 2 mA), inter-leaved with sham (i.e. control) trials [189]. Stimulation increased cross-frequency coupling on higher-load trials at stimulated sites (see figure 3(c)) and reduced behavioral response times, demonstrating a causal oscillatory PFC mechanism supporting working memory. Based on observations of low-frequency oscillatory connectivity in the frontoparietal network, the same group stimulated lateral PFC and PPC electrodes at individuals’ peak interaction frequency (4–12 Hz) to manipulate connectivity during working memory. They found that in-phase stimulation increased functional connectivity between stimulated sites and increased task accuracy, whereas anti-phase stimulation increased the lag time between regions and decreased accuracy [190]. Together, these finding reveal an oscillatory mechanism governing causal contributions of PFC and frontoparietal networks to working memory.
The outcomes are highly variable in the more-common studies of episodic memory (reviewed in [191–193]). Whereas direct iES of the hippocampus has been shown to disrupt memory performance [194, 195], frequency-tuned stimulation of regions with connections to the hippocampus has been shown to improve performance. Indeed, Solomon and colleagues observed that the strength of short-range theta connectivity between MTL sub-regions predicted theta stimulation-induced theta power [165]. Assuming episodic memory relies on theta synchrony within MTL networks (see figure 4(c)), then stimulation which increases MTL theta synchrony should improve performance. Accordingly, these studies provide converging evidence that frequency-specific hippocampal connections underlie distinct mnemonic processes.
During memory encoding (section 3.2), Jun and colleagues found that iES of the hippocampus induced theta power and improved subsequent retrieval of associations, but not items [196, 197], illuminating the need to target function in a task-appropriate manner. Fried, Suthana, and colleagues found that stimulation of the adjacent entorhinal cortex (1–2 mA) reset hippocampal theta oscillations and improved retrieval of both locations and items [198, 199]. In contrast, low-amplitude (0.1 mA) stimulation increased hippocampal ERPs but had no effect on behavior [200]. By stimulating the fornix, a white matter structure connecting the hippocampus and subcortical regions, in the theta band prior to encoding, Koubeissi and colleagues observed increased hippocampal and posterior cingulate evoked responses and improved subsequent verbal recall [201]. Likewise, iES disruption (100 Hz) of posterior cingulate during encoding was found to de-couple theta (2–10 Hz) and broadband gamma activities in the hippocampus and impair subsequent verbal recall [202]. These findings demonstrate that the location, frequency, amplitude, and task-guided nature of stimulation are paramount, and that synchronous theta oscillations within MTL and in MTL-connected networks play a causal role in memory encoding.
Finally, by applying iES during retrieval (section 3.3), one study suggested that network connectivity more broadly governs the critical contribution of MTL and MTL-connected networks to episodic memory. Fell and colleagues stimulated MTL at both encoding and retrieval in the gamma band (40 Hz), which induced gamma connectivity between the hippocampus and rhinal cortex and improved verbal recall [203]. In a study of network connectivity, Kim and colleagues found that decoupling two key memory regions (PFC, PPC, MTL, and/or temporal cortex) via anti-phase stimulation between sites in the theta band selectively impaired spatial memory retrieval [204]. Although more research is needed, these findings suggest that synchronized theta and gamma activities in key memory regions and networks are causally involved in episodic memory function and may be modulated to enhance performance.
5.3. Conclusions
Since the pioneering work of Penfield, clinicians and researchers have employed iES as a tool to test the causality of precise brain regions in cognitive functions. Outcomes are diverse, linking phenomena ranging from laughter to memory to out-of-body experiences directly to different neural regions and networks with millimeter precision. Recently, researchers have used a comparable technique, but employed band-limited stimulation at frequencies associated with specific functions, to test the causality of spatiotemporally precise neural activity in cognitive functions. Insights from this approach illuminate the causal role of theta oscillations in coordinating key memory regions during working and episodic memory, informing basic science and translational applications aimed at intervention. Although some studies exist which have applied these insights to design brain-machine interface and closed-loop systems to restore or enhance function [205–207], this work is in its infancy.
Like research using iEEG to understand how different cognitive functions are implemented by the human brain, additional iES research is needed to better understand nuances in causal relationships between specific brain structures and functions and cognitive behaviors. Indeed, a recent large-scale study demonstrated that iES mapping elicits effects based on intrinsic functional-anatomical architecture, with more diverse effects (e.g. facilitation, disruption) observed in networks higher in the cortical hierarchy [169]. Beyond establishing causal links, future iES studies may aim to lend insight into the mechanistic differences reflected by facilitation versus disruption of specific behaviors. Thus, future research should further delineate whether and how different neural mechanisms are causally involved in specific neurocognitive functions.
6. Discussion
By measuring spatiotemporally precise neural activity with single-trial precision, iEEG tracks neurocognitive processes with high resolution as they unfold in real time. Research using iEEG to study human cognition thus yields insights to the field of cognitive neuroscience that extend beyond what can be known from noninvasive approaches. In this review, we delineated insights into auditory perception and prediction (section 2), working and episodic memory (section 3), and internally oriented cognition (section 4) from studies using ECoG and sEEG in humans. We showcased these fields as examples but note that the unique contributions of iEEG to advancing our understanding of human cognition are directly applicable to numerous other fields. By demonstrating not just where and when, but how, cognitive processes are implemented in the human brain, these data inform unique mechanistic explanations of the neural basis of cognition. We closed by highlighting the use of iES as a tool to infer the causality of spatially precise brain regions and spatiotemporally precise neural activity in specific cognitive functions (section 5). By engineering the brain and observing changes in iEEG measures and behavioral outputs, iES data address the fundamental issue of causality. Intracranial approaches will further inform questions central to basic science and carry immense translational potential to inform successful intervention.
6.1. Limitations
Here, despite the mechanistic insights obtained from intracranial research, we outline several limitations of iEEG as a technique to understand human cognition. First, although iEEG recordings have high spatial precision, they have limited spatial coverage. Electrode placement is driven solely by clinical needs to sample brain regions which are common sources of epilepsy. Accordingly, some areas may be over-sampled and other areas under-sampled. Studies of functional connectivity may be particularly affected by this caveat, as often not all regions of interest are available within the same patient cohort. A recent study addressed this issue. Based on the assumption that individuals’ brains exhibit similar correlational structure, Owen and colleagues developed a model-based approach to infer neural activity at non-sampled sites [163]. Intriguingly, whereas reconstruction of full-brain neural activity was found to be reliable at all frequencies, it was most robust in the theta band—which, as we describe in this review, is critically involved in numerous cognitive processes.
Second, iEEG studies are always performed in brains of epileptic patients. Typically, patients included in iEEG studies suffer from focal epilepsy, and epileptogenic sites are normally excluded from analyses. Data reported in iEEG studies of cognition are thought to reflect healthy tissue and neural responses. Rossini and colleagues provided strong evidence for this critical point by showing that epileptic seizures do not contribute to focal brain injury outside epileptic regions [4]. Nevertheless, imaging studies have reported gray matter damage in the autonomic control network of some of these patients [208]. Intracranial researchers must therefore meticulously control for activity related to pathology, for instance, by removing any abnormal data or comparing iEEG data to independent samples of non-clinical participants using noninvasive methods.
6.2. Future directions and open questions
Despite a plethora of studies investigating the neural origins of macroscopic signals as assessed via iEEG (i.e. ERPs and HFA), the exact nature of these signals remains elusive. For instance, HFA is thought to represent a highly spatiotemporally precise signal, reflective of neuronal firing and BOLD response modulations [6–13]. However, recent invasive evidence from monkeys suggests that the neural origins of HFA are more nuanced; HFA originates mainly from deep cortical layers and includes not only action potentials, but also dendritic processes [209]. Accordingly, the spatial extent and laminar origin of HFA responses should be the subject of future investigations. We further note that HFA is a broadband response, typically measured between 70 and 300 Hz, that reflects neuronal activity as opposed to narrowband oscillations. Studies vary widely in which HFA range is chosen for analysis, however, whether different sub-bands serve different functional roles remains unknown.
Finally, there are burgeoning approaches which further increase spatiotemporal precision compared to iEEG. For instance, a few international research groups have developed techniques to record single neurons by attaching micro-wires to the ends of sEEG probes (i.e. microelectrodes) to track both neurons and local field potentials from the terminal end of the depth probes [3, 191, 198, 199, 210, 211]. As the use of intracranial techniques to study human cognition continues to increase, future research may use these data to address questions regarding the precise relationship between HFA and human neuronal responses, among other things.
6.3. Neuroengineering implications
In addition to advancing basic science, there are important practical implications of understanding how cognitive processes are implemented in the human brain. For instance, insights from iES studies probing the causal mechanisms of memory have been applied to design brain-machine interface and closed-loop systems to enhance memory function [204–206]. However, mixed outcomes illuminate the need to gain deeper understanding of nuanced relationships between specific brain structures and functions and cognitive behaviors to develop reliable systems. Thus, a major neuroengineering implication of iEEG and iES studies is the design of neuroprosthetic devices to restore and enhance cognitive function. Considering the perception of external stimuli, predictive cues are embedded in our daily lives, such as when listening to music [212] or speech [213], and therefore facilitate perception of the environment. Understanding how the human brain uses these cues to process sensory information may inform the design of neuroprosthetic devices that facilitate perception of streams of sensory information, among other things.
Moreover, detection of sensory rules is disrupted in patients with disorders of consciousness [22] and in a host of psychiatric disorders, such as schizophrenia [214]. A detailed characterization of the neural network underlying regularity detection can thus help in the design of diagnostic tests for assessing residual levels of consciousness [22] and psychiatric disorders [214]. Therefore, another major neuroengineering implication of iEEG and iES studies is the design of diagnostic tests for patients with different disorders, including but not limited to those affecting consciousness, memory, and self-referential thought. Considering internally oriented thinking in the absence of external cues, understanding of the neural signatures underlying various types of internal cognition could help facilitate decoding of ongoing thoughts. Reliably achieving this feat could provide communication ability to patients with language and speech disabilities, or even those with locked-in syndrome.
In conclusion, iEEG and iES have provided unique insights into fundamental cognitive processes in humans and promises to inform future research that seeks to understand, restore, and enhance human cognition.
Acknowledgments
We thank Dr. Anna Jafarpour for assistance planning this review. This work was funded by the National Institute of Neurological Disorders and Stroke (Grant Nos. 2R37NS21135, K99NS115918), National Institute of Mental Health (Conte Center P50 MH109429), James S. McDonnell Foundation (Grant No. 220020448), and Swiss National Science Foundation (Grant Nos. 320030_188737, P300PA_174451). A.T. is supported by the Interfaculty Research Cooperation ‘Decoding Sleep: From Neurons to Health & Mind’ of the University of Bern.
Footnotes
Conflicts of interest
None.
References
- [1].Norman Y, Yeagle EM, Khuvis S, Harel M, Mehta AD and Malach R 2019. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans Science 365 eaax1030. [DOI] [PubMed] [Google Scholar]
- [2].Kwan P, Schachter SC and Brodie MJ 2011. Drug-resistant epilepsy N. Engl. J. Med 365 919–26 [DOI] [PubMed] [Google Scholar]
- [3].Chiong W, Leonard MK and Chang EF 2017. Neurosurgical patients as human research subjects: ethical considerations in intracranial electrophysiology research Neurosurgery 83 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rossini L et al. 2017. Seizure activity per se does not induce tissue damage markers in human neocortical focal epilepsy Ann. Neurol 82 331–41 [DOI] [PubMed] [Google Scholar]
- [5].Ball T, Kern M, Mutschler I, Aertsen A and Schulze-Bonhage A 2009. Signal quality of simultaneously recorded invasive and non-invasive EEG Neuroimage 46 708–16 [DOI] [PubMed] [Google Scholar]
- [6].Ray S, Crone NE, Niebur E, Franaszczuk PJ and Hsiao SS 2008. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography J. Neurosci 28 11526–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rich EL and Wallis JD 2017. Spatiotemporal dynamics of information encoding revealed in orbitofrontal high-gamma Nat. Commun 8 1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Watson BO, Ding M and Buzśaki G 2018. Temporal coupling of field potentials and action potentials in the neocortex Eur. J. Neurosci 48 2482–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I and Malach R 2007. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations Curr. Biol 17 1275–85 [DOI] [PubMed] [Google Scholar]
- [10].Conner CR, Ellmore TM, Pieters TA, Di Sano MA and Tandon N 2011. Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans J. Neurosci 31 12855–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hermes D, Miller KJ, Vansteensel MJ, Aarnoutse EJ, Leijten FSS and Ramsey NF 2012. Neurophysiologic correlates of fMRI in human motor cortex Hum. Brain Mapp 33 1689–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tasawoor Kanth S and Ray S 2020. Electrocorticogram (ECoG) is highly informative in primate visual cortex J. Neurosci 40 2430–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fedele T, Tzovara A, Steiger B, Hilfiker P, Grunwald T, Stieglitz L, Jokeit H and Sarnthein J 2020. The relation between neuronal firing, local field potentials and hemodynamic activity in the human amygdala in response to aversive dynamic visual stimuli Neuroimage 213 116705. [DOI] [PubMed] [Google Scholar]
- [14].Bar M 2009. Predictions: a universal principle in the operation of the human brain. Introduction Philos. Trans. R. Soc. Lond. B. Biol. Sci 364 1181–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mumford D 1992. On the computational architecture of the neocortex - II the role of cortico-cortical loops Biol. Cybern 66 241–51 [DOI] [PubMed] [Google Scholar]
- [16].Phillips HN, Blenkmann A, Hughes LE, Kochen S, Bekinschtein TA, Cam CAN and Rowe JB 2016. Convergent evidence for hierarchical prediction networks from human electrocorticography and magnetoencephalography Cortex 82 192–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garrido MI, Kilner JM, Stephan KE and Friston KJ 2009. The mismatch negativity: a review of underlying mechanisms Clin. Neurophysiol 120 453–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Näätänen R, Gaillard AWK and Mäntysalo S 1978. Early selective-attention effect on evoked potential reinterpreted Acta Psychol. 42 313–29 [DOI] [PubMed] [Google Scholar]
- [19].Opitz B, Rinne T, Mecklinger A, Von Cramon DY and Schröger E 2002. Differential contribution of frontal and temporal cortices to auditory change detection: FMRI and ERP results Neuroimage 15 167–74 [DOI] [PubMed] [Google Scholar]
- [20].Chennu S, Noreika V, Gueorguiev D, Shtyrov Y, Bekinschtein TA and Henson R 2016. Silent expectations: dynamic causal modeling of cortical prediction and attention to sounds that weren’t J. Neurosci 36 8305–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T and Kilner JM 2008. The functional anatomy of the MMN: a DCM study of the roving paradigm Neuroimage 42 936–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, Massimini M, Litvak V, Laureys S and Friston K 2011. Preserved feedforward but impaired top-down processes in the vegetative state Science 332 858–62 [DOI] [PubMed] [Google Scholar]
- [23].Halgren E, Baudena P, Clarke JM, Heit G, Liégeois C, Chauvel P and Musolino A 1995. Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe Electroencephalogr. Clin. Neurophysiol 94 191–220 [DOI] [PubMed] [Google Scholar]
- [24].Halgren E, Squires NK, Wilson CL, Rohrbaugh JW, Babb TL and Crandall PH 1980. Endogenous potentials generated in the human hippocampal formation and amygdala by infrequent events Science 210 803–5 [DOI] [PubMed] [Google Scholar]
- [25].Rosburg T, Trautner P, Dietl T, Korzyukov OA, Boutros NN, Schaller C, Elger CE and Kurthen M 2005. Subdural recordings of the mismatch negativity (MMN) in patients with focal epilepsy Brain 128 819–28 [DOI] [PubMed] [Google Scholar]
- [26].Edwards E, Soltani M, Deouell LY, Berger MS and Knight RT 2005. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex J. Neurophysiol 94 4269–80 [DOI] [PubMed] [Google Scholar]
- [27].Dürschmid S, Edwards E, Reichert C, Dewar C, Hinrichs H, Heinze H-J, Kirsch HE, Dalal SS, Deouell LY and Knight RT 2016. Hierarchy of prediction errors for auditory events in human temporal and frontal cortex Proc. Natl Acad. Sci 113 6755–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Leonard MK, Baud MO, Sjerps MJ and Chang EF 2016. Perceptual restoration of masked speech in human cortex Nat. Commun 7 13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blenkmann AO et al. 2019. Auditory deviance detection in the human insula: an intracranial EEG study Cortex 121 189–200 [DOI] [PubMed] [Google Scholar]
- [30].El Karoui I et al. 2015. Event-related potential, time-frequency, and functional connectivity facets of local and global auditory novelty processing: an intracranial study in humans Cereb. Cortex 25 4203–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nourski KV, Steinschneider M, Rhone AE and Kawasaki H 2018. Processing of Auditory Novelty across the Cortical Hierarchy: An Intracranial Electrophysiology Study Neuroimage vol 183 412–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L and Naccache L 2009. Neural signature of the conscious processing of auditory regularities Proc. Natl Acad. Sci 106 1672–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nourski KV, Steinschneider M, Rhone AE, Kawasaki H, Howard MA and Banks MI 2018. Auditory predictive coding across awareness states under anesthesia: an intracranial electrophysiology study J. Neurosci 38 8441–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ulanovsky N, Las L, Farkas D and Nelken I 2004. Multiple time scales of adaptation in auditory cortex neurons J. Neurosci 24 10440–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Barascud N, Pearce MT, Griffiths TD, Friston KJ and Chait M 2016. Brain responses in humans reveal ideal observer-like sensitivity to complex acoustic patterns Proc. Natl Acad. Sci 113 E616–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tzovara A, Simonin A, Oddo M, Rossetti AO and De Lucia M 2015. Neural detection of complex sound sequences in the absence of consciousness Brain 138 1160–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hughes HC, Darcey TM, Barkan HI, Williamson PD, Roberts DW and Aslin CH 2001. Responses of human auditory association cortex to the omission of an expected acoustic event Neuroimage 13 1073–89 [DOI] [PubMed] [Google Scholar]
- [38].Fonken YM, Mukerji A, Jimenez R, Lin J, Brunner P, Schalk G and Knight RT 2019. Unexpected sound omissions are signaled in human posterior superior temporal gyrus: an intracranial study bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chouiter L, Tzovara A, Dieguez S, Annoni J-M, Magezi D, De Lucia M and Spierer L 2015. Experience-based auditory predictions modulate brain activity to silence as do real sounds J. Cogn. Neurosci 27 1968–80 [DOI] [PubMed] [Google Scholar]
- [40].Berlot E, Formisano E and De Martino F 2018. Mapping frequency-specific tone predictions in the human auditory cortex at high spatial resolution J. Neurosci 38 4934–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Duerschmid S, Reichert C, Hinrichs H, Heinze H-J, Kirsch HE, Knight RT and Deouell LY 2018. Direct evidence for prediction signals in frontal cortex independent of prediction error Cereb. Cortex 29 4530–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Auksztulewicz R, Schwiedrzik CM, Thesen T, Doyle W, Devinsky O, Nobre AC, Schroeder CE, Friston KJ and Melloni L 2018. Not all predictions are equal: “what” and “when” predictions modulate activity in auditory cortex through different mechanisms J. Neurosci 38 8680–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kikuchi Y et al. 2017. Sequence learning modulates neural responses and oscillatory coupling in human and monkey auditory cortex PLoS Biol. 15 e2000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Holdgraf CR, de Heer W, Pasley B, Rieger J, Crone N, Lin JJ, Knight RT and Theunissen FE 2016. Rapid tuning shifts in human auditory cortex enhance speech intelligibility Nat. Commun 7 13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jafarpour A, Piai V, Lin JJ and Knight RT 2017. Human hippocampal pre-activation predicts behavior Sci. Rep 7 5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P and Friston KJ 2012. Canonical microcircuits for predictive coding Neuron 76 695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tulving E 1972. Episodic and semantic memory Organ. Mem 1 381–403 [Google Scholar]
- [48].Baddeley A 1992. Working memory Science 255 556–9 [DOI] [PubMed] [Google Scholar]
- [49].Miller GA 1956. The magical number seven, plus or minus two: some limits on our capacity for processing information Psychol. Rev 101 343–52 [DOI] [PubMed] [Google Scholar]
- [50].Cowan N 2000. The magical number 4 in short-term memory: a reconsideration of mental storage capacity Behav. Brain Sci 24 87–185 [DOI] [PubMed] [Google Scholar]
- [51].Brady TF, Konkle T, Alvarez GA and Oliva A 2008. Visual long-term memory has a massive storage capacity for object details Proc. Natl Acad. Sci 105 14325–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fukuda K and Vogel EK 2019. Visual short-term memory capacity predicts the “bandwidth” of visual long-term memory encoding Mem. Cogn 47 1481–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A and Kahana MJ 2003. Gamma oscillations correlate with working memory load in humans Cereb. Cortex 13 1369–74 [DOI] [PubMed] [Google Scholar]
- [54].Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O and Lachaux JP 2007. Neural correlates of consolidation in working memory Hum. Brain Mapp 28 183–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Van Vugt MK, Schulze-Bonhage A, Litt B, Brandt A and Kahana MJ 2010. Hippocampal gamma oscillations increase with memory load J. Neurosci 30 2694–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dickerson BC and Eichenbaum H 2010. The episodic memory system: neurocircuitry and disorders Neuropsychopharmacology 35 86–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Griffiths BJ et al. 2019. Directional coupling of slow and fast hippocampal gamma with neocortical alpha/beta oscillations in human episodic memory Proc. Natl Acad. Sci 116 21834–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE and Fell J 2010. Cross-frequency coupling supports multi-item working memory in the human hippocampus Proc. Natl Acad. Sci 107 3228–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Axmacher N, Mormann F, Cohen MX and Elger CE 2007. Sustained neural activity patterns during working memory in the human medial temporal lobe J. Neurosci 27 7807–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Axmacher N, Elger CE and Fell J 2009. Working memory-related hippocampal deactivation interferes with long-term memory formation J. Neurosci 29 1052–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kleen JK, Testorf ME, Roberts DW, Scott RC, Jobst BJ and Holmes GL 2016. Oscillation phase locking and late ERP components of intracranial hippocampal recordings correlate to patient performance in a working memory task Front. Hum. Neurosci 10 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ni B, Wu R, Yu T, Zhu H, Li Y and Liu Z 2017. Role of the hippocampus in distinct memory traces: timing of match and mismatch enhancement revealed by intracranial recording Neurosci. Bull 33 664–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Khursheed F, Tandon N, Tertel K, Pieters TA, Disano MA and Ellmore TM 2011. Frequency-specific electrocorticographic correlates of working memory delay period fMRI activity Neuroimage 56 1773–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Noy N et al. 2015. Intracranial recordings reveal transient response dynamics during information maintenance in human cerebral cortex Hum. Brain Mapp 36 3988–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kambara T, Brown EC, Jeong JW, Ofen N, Nakai Y and Asano E 2017. Spatio-temporal dynamics of working memory maintenance and scanning of verbal information Clin. Neurophysiol 128 882–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Buzsaki G and Draughn A 2004. Neuronal oscillations in cortical networks Science 304 1926–30 [DOI] [PubMed] [Google Scholar]
- [67].Gyorgy B 2002. Theta oscillations in the hippocampus Neuron 33 325–40 [DOI] [PubMed] [Google Scholar]
- [68].Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR and Lisman JE 2001. Gating of human theta oscillations by a working memory task J. Neurosci 21 3175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Raghavachari S et al. 2013. Theta oscillations in human cortex during a working-memory task: evidence for local generators J. Neurophysiol 95 1630–8 [DOI] [PubMed] [Google Scholar]
- [70].Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, Spencer DD and Constable RT 2008. Effects of working memory load on oscillatory power in human intracranial EEG Cereb. Cortex 18 1843–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Brzezicka A, Kamiński J, Reed C, Chung J, Mamelak A and Rutishauser U 2019. Working memory load related theta power decreases in dorsolateral prefrontal cortex predict individual differences in performance J. Cogn. Neurosci 31 1290–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hanslmayr S, Staresina BP and Bowman H 2016. Oscillations and episodic memory: addressing the synchronization/desynchronization conundrum Trends Neurosci. 39 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Leszczyński M, Fell J and Axmacher N 2015. Rhythmic working memory activation in the human hippocampus Cell Rep. 13 1272–82 [DOI] [PubMed] [Google Scholar]
- [74].Chaieb L, Leszczynski M, Axmacher N, Hohne M, Elger CE and Fell J 2015. Theta-gamma phase-phase coupling during working memory maintenance in the human hippocampus Cogn. Neurosci 6 149–57 [DOI] [PubMed] [Google Scholar]
- [75].Johnson EL, Adams JN, Solbakk A, Endestad T, Larsson G, Ivanovic J, Meling TR, Lin JJ and Knight RT 2018. Dynamic frontotemporal systems process space and time in working memory PLoS Biol. 16 e2004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM and Knight RT 2006. High gamma power is phase-locked to theta oscillations in human neocortex Science 313 1626–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Maris E, van Vugt M and Kahana M 2011. Spatially distributed patterns of oscillatory coupling between high-frequency amplitudes and low-frequency phases in human iEEG Neuroimage 54 836–50 [DOI] [PubMed] [Google Scholar]
- [78].van der Meij R, Kahana M and Maris E 2012. Phase-amplitude coupling in human electrocorticography is spatially distributed and phase diverse J. Neurosci 32 111–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Johnson EL, King-Stephens D, Weber PB, Laxer KD, Lin JJ and Knight RT 2019. Spectral imprints of working memory for everyday associations in the frontoparietal network Front. Syst. Neurosci 12 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang Q, van Vugt M, Borst JP and Anderson JR 2018. Mapping working memory retrieval in space and in time: a combined electroencephalography and electrocorticography approach Neuroimage 174 472–84 [DOI] [PubMed] [Google Scholar]
- [81].Johnson EL and Knight RT 2015. Intracranial recordings and human memory Curr. Opin. Neurobiol 31 18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR and Kahana MJ 2014. Human intracranial high-frequency activity maps episodic memory formation in space and time Neuroimage 85 834–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kucewicz MT. et al. Human verbal memory encoding is hierarchically distributed in a continuous processing stream. eNeuro. 2019;6:1. doi: 10.1523/ENEURO.0214-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kucewicz MT et al. 2017. Dissecting gamma frequency activity during human memory processing Brain 140 1337–50 [DOI] [PubMed] [Google Scholar]
- [85].Johnson EL, Tang L, Yin Q, Asano E and Ofen N 2018. Direct brain recordings reveal prefrontal cortex dynamics of memory development Sci. Adv 4 aat3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ofen N, Tang L, Yu Q and Johnson EL 2019. Memory and the developing brain: from description to explanation with innovation in methods Dev. Cogn. Neurosci 36 100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Burke JF, Zaghloul KA, Jacobs J, Williams RB, Sperling MR, Sharan AD and Kahana MJ 2013. Synchronous and asynchronous theta and gamma activity during episodic memory formation J. Neurosci 33 292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Solomon EA. et al. Widespread theta synchrony and high-frequency desynchronization underlies enhanced cognition. Nat. Commun. 2017;8:1704. doi: 10.1038/s41467-017-01763-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Serruya MD, Sederberg PB and Kahana MJ 2014. Power shifts track serial position and modulate encoding in human episodic memory Cereb. Cortex 24 403–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Long NM, Burke JF and Kahana MJ 2014. Subsequent memory effect in intracranial and scalp EEG Neuroimage 84 488–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Long NM and Kahana MJ 2015. Successful memory formation is driven by contextual encoding in the core memory network Neuroimage 119 332–7 [DOI] [PubMed] [Google Scholar]
- [92].Greenberg JA, Burke JF, Haque R, Kahana MJ and Zaghloul KA 2015. Decreases in theta and increases in high frequency activity underlie associative memory encoding Neuroimage 114 257–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kragel JE et al. 2017. Similar patterns of neural activity predict memory function during encoding and retrieval Neuroimage 155 60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Solomon EA et al. 2019. Dynamic theta networks in the human medial temporal lobe support episodic memory Curr. Biol 29 1100–111.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Matsumoto JY et al. 2013. Network oscillations modulate interictal epileptiform spike rate during human memory Brain 136 2444–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sheehan TC, Sreekumar V, Inati SK and Zaghloul KA 2018. Signal complexity of human intracranial EEG tracks successful associative memory formation across individuals J. Neurosci 38 1744–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Fellner M-C, Gollwitzer S, Rampp S, Kreiselmeyer G, Bush D, Diehl B, Axmacher N, Hamer H and Hanslmayr S 2019. Spectral fingerprints or spectral tilt? Evidence for distinct oscillatory signatures of memory formation PLoS Biol. 17 e3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Herweg NA, Solomon EA and Kahana MJ 2019. Theta oscillations in human memory Trends Cogn. Sci 24 208–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Vaz AP, Yaffe RB, Wittig JH, Inati SK, Zaghloul KA, Branch SN, Scientist M and Program T 2017. Dual origins of measured phase-amplitude coupling reveal distinct neural mechanisms underlying episodic memory in the human cortex Neuroimage 148 148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lega BC, Jacobs J and Kahana M 2012. Human hippocampal theta oscillations and the formation of episodic memories Hippocampus 22 748–61 [DOI] [PubMed] [Google Scholar]
- [101].Lin JJ, Rugg MD, Das S, Stein J, Rizzuto DS, Kahana MJ and Lega BC 2017. Theta band power increases in the posterior hippocampus predict successful episodic memory encoding in humans Hippocampus 27 1040–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lega B, Burke J, Jacobs J and Kahana MJ 2016. Slow-theta-to-gamma phase-amplitude coupling in human hippocampus supports the formation of new episodic memories Cereb. Cortex 26 268–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Young JJ, Rudebeck PH, Marcuse LV, Fields MC, Yoo JY, Panov F, Ghatan S, Fazl A, Mandelbaum S and Baxter MG 2018. Theta band network supporting human episodic memory is not activated in the seizure onset zone Neuroimage 183 565–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Fell J, Klaver P, Elfadil H, Schaller C, Elger CE and Fernández G 2003. Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma synchronization? Eur. J. Neurosci 17 1082–8 [DOI] [PubMed] [Google Scholar]
- [105].Gruber MJ, Hsieh L-T, Staresina BP, Elger CE, Fell J, Axmacher N and Ranganath C 2018. Theta phase synchronization between the human hippocampus and prefrontal cortex increases during encoding of unexpected information: a case study J. Cogn. Neurosci 30 1646–56 [DOI] [PubMed] [Google Scholar]
- [106].Sweeney-Reed CM et al. 2016. Pre-stimulus thalamic theta power predicts human memory formation Neuroimage 138 100–8 [DOI] [PubMed] [Google Scholar]
- [107].Sweeney-Reed CM. et al. Thalamic theta phase alignment predicts human memory formation and anterior thalamic cross-frequency coupling. eLife. 2015;4:e07578. doi: 10.7554/eLife.07578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Höhne M, Jahanbekam A, Bauckhage C, Axmacher N and Fell J 2016. Prediction of successful memory encoding based on single-trial rhinal and hippocampal phase information Neuroimage 139 127–35 [DOI] [PubMed] [Google Scholar]
- [109].Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE and Axmacher N 2011. Medial temporal theta/alpha power enhancement precedes successful memory encoding: evidence based on intracranial EEG J. Neurosci 31 5392–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sweeney-Reed CM. et al. Corticothalamic phase synchrony and cross-frequency coupling predict human memory formation. eLife. 2014;3:e05352. doi: 10.7554/eLife.05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tulving E and Thomson DM 1973. Encoding specificity and retrieval processes in episodic memory Psychol. Rev 80 352–73 [Google Scholar]
- [112].Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, Litt B, Brandt A and Kahana MJ 2007. Gamma oscillations distinguish true from false memories Psychol. Sci 18 927–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Long NM et al. 2017. Contextually mediated spontaneous retrieval is specific to the hippocampus Curr. Biol 27 1074–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Merkow MB, Burke JF and Kahana MJ 2015. The human hippocampus contributes to both the recollection and familiarity components of recognition memory Proc. Natl. Acad. Sci 112 14378–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Staresina BP, Michelmann S, Bonnefond M, Jensen O, Axmacher N and Fell J 2016. Hippocampal pattern completion is linked to gamma power increases and alpha power decreases during recollection eLife 5 e17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Pacheco Estefan D,Sánchez-Fibla M, Duff A, Principe A, Rocamora R, Zhang H, Axmacher N and Verschure PFMJ 2019. Coordinated representational reinstatement in the human hippocampus and lateral temporal cortex during episodic memory retrieval Nat. Commun 10 2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fell J, Wagner T, Staresina BP, Ranganath C, Elger CE and Axmacher N et al. 2016. Rhinal-hippocampal information flow reverses between memory encoding and retrieval Neural Information Processing, ed Hirose A, Ozawa S, Doya K, Ikeda K, Lee M and Liu D (Berlin: Springer; ) pp 105–14 [Google Scholar]
- [118].Herweg NA, Sharan AD, Sperling MR, Brandt A, Schulze-Bonhage A and Kahana MJ 2020. Reactivated spatial context guides episodic recall J. Neurosci 40 2119–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kragel JE, Vanhaerents S, Templer JW, Schuele S, Rosenow JM, Nilakantan AS and Bridge DJ 2020. Hippocampal theta coordinates memory processing during visual exploration eLife 9 e52108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Watrous AJ, Tandon N, Conner CR, Pieters T and Ekstrom AD 2013. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval Nat. Neurosci 16 349–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Bauch EM, Bunzeck N, Hinrichs H, Schmitt FC, Voges J, Heinze HJ and Zaehle T 2018. Theta oscillations underlie retrieval success effects in the nucleus accumbens and anterior thalamus: evidence from human intracranial recordings Neurobiol. Learn. Mem 155 104–12 [DOI] [PubMed] [Google Scholar]
- [122].Lohnas LJ, Duncan K, Doyle WK, Thesen T, Devinsky O and Davachi L 2018. Time-resolved neural reinstatement and pattern separation during memory decisions in human hippocampus Proc. Natl. Acad. Sci 115 E7418–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Stevenson RF, Zheng J, Mnatsakanyan L, Vadera S, Knight RT, Lin JJ and Yassa MA 2018. Hippocampal CA1 gamma power predicts the precision of spatial memory judgments Proc. Natl Acad. Sci 115 10148–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zheng J, Stevenson RF, Mander BA, Mnatsakanyan L, Hsu FPK, Vadera S, Knight RT, Yassa MA and Lin JJ 2019. Multiplexing of theta and alpha rhythms in the amygdala-hippocampal circuit supports pattern separation of emotional information Neuron 102 887–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].El-Kalliny MM, Wittig JH, Sheehan TC, Sreekumar V, Inati SK and Zaghloul KA 2019. Changing temporal context in human temporal lobe promotes memory of distinct episodes Nat. Commun 10 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Axmacher N, Elger CE and Fell J 2008. Ripples in the medial temporal lobe are relevant for human memory consolidation Brain 131 1806–17 [DOI] [PubMed] [Google Scholar]
- [127].Vaz AP, Inati SK, Brunel N and Zaghloul KA 2019. Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory Science 363 975–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Crone N 1998. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band Brain 121 2301–15 [DOI] [PubMed] [Google Scholar]
- [129].Liégeois-Chauvel C, Lorenzi C, Trébuchon A, Régis J and Chauvel P 2004. Temporal envelope processing in the human left and right auditory cortices Cereb. Cortex 14 731–40 [DOI] [PubMed] [Google Scholar]
- [130].Tang H, Buia C, Madhavan R, Crone NE, Madsen JR, Anderson WS and Kreiman G 2014. Spatiotemporal dynamics underlying object completion in human ventral visual cortex Neuron 83 736–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Beauchamp MS, Sun P, Baum SH, Tolias AS and Yoshor D 2012. Electrocorticography links human temporoparietal junction to visual perception Nat. Neurosci 15 957–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M and Aron AR 2009. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses J. Neurosci 29 12675–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Margulies DS et al. 2016. Situating the default-mode network along a principal gradient of macroscale cortical organization Proc. Natl Acad. Sci 113 12574–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Gusnard DA and Raichle ME 2001. Searching for a baseline: functional imaging and the resting human brain Nat. Rev. Neurosci 2 685–94 [DOI] [PubMed] [Google Scholar]
- [135].Buckner RL, Andrews-Hanna JR and Schacter DL 2008. The brain’s default network: anatomy, function, and relevance to disease Ann. N. Y. Acad. Sci 1124 1–38 [DOI] [PubMed] [Google Scholar]
- [136].Fox KCR, Foster BL, Kucyi A, Daitch AL and Parvizi J 2018. Intracranial electrophysiology of the human default network Trends Cogn. Sci 22 307–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST and Macrae CN 2007. Wandering minds: the default network and stimulus-independent thought Science 315 393–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Christoff K, Gordon AM, Smallwood J, Smith R and Schooler JW 2009. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering Proc. Natl Acad. Sci 106 8719–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Greicius MD, Krasnow B, Reiss AL and Menon V 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis Proc. Natl Acad. Sci 100 253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Cole MW, Bassett DS, Power JD, Braver TS and Petersen SE 2014. Intrinsic and task-evoked network architectures of the human brain Neuron 83 238–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Thomas Yeo BT et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity J. Neurophysiol 106 1125–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kucyi A, Schrouff J, Bickel S, Foster BL, Shine JM and Parvizi J 2018. Intracranial electrophysiology reveals reproducible intrinsic functional connectivity within human brain networks J. Neurosci 38 4230–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bettus G. et al. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PLoS One. 2011;6:e20071. doi: 10.1371/journal.pone.0020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kucyi A. et al. Electrophysiological dynamics of antagonistic brain networks reflect attentional fluctuations. Nat. Commun. 2020;11:325. doi: 10.1038/s41467-019-14166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC and Raichle ME 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks Proc. Natl Acad. Sci 102 9673–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Dastjerdi M et al. 2011. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex Proc. Natl Acad. Sci 108 3023–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Daitch AL and Parvizi J 2018. Spatial and temporal heterogeneity of neural responses in human posteromedial cortex Proc. Natl Acad. Sci 115 4785–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Foster BL, Rangarajan V, Shirer WR and Parvizi J 2015. Intrinsic and task-dependent coupling of neuronal population activity in human parietal cortex Neuron 86 578–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Miller KJ, Weaver KE and Ojemann JG 2009. Direct electrophysiological measurement of human default network areas Proc. Natl Acad. Sci 106 12174–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Andrews-Hanna JR, Smallwood J and Spreng RN 2014. The default network and self-generated thought: component processes, dynamic control, and clinical relevance Ann. N. Y. Acad. Sci 1316 29–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Foster BL, Kaveh A, Dastjerdi M, Miller KJ and Parvizi J 2013. Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval J. Neurosci 33 10439–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Canolty RT and Knight RT 2010. The functional role of cross-frequency coupling Trends Cogn. Sci 14 506–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Killingsworth MA and Gilbert DT 2010. A wandering mind is an unhappy mind Science 330 932. [DOI] [PubMed] [Google Scholar]
- [154].Christoff K, Irving ZC, Fox KCR, Spreng RN and Andrews-Hanna JR 2016. Mind-wandering as spontaneous thought: a dynamic framework Nat. Rev. Neurosci 17 718–31 [DOI] [PubMed] [Google Scholar]
- [155].Seli P, Risko EF, Smilek D and Schacter DL 2016. Mind-wandering with and without intention Trends Cogn. Sci 20 605–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Foster BL, Dastjerdi M and Parvizi J 2012. Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing Proc. Natl Acad. Sci 109 15514–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Kam JWY, Lin JJ, Solbakk A-K, Endestad T, Larsson PG and Knight RT 2019. Default network and frontoparietal control network theta connectivity supports internal attention Nat. Hum. Behav 3 1263–70 [DOI] [PubMed] [Google Scholar]
- [158].Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW and Schacter DL 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition Neuroimage 53 303–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW and Christoff K 2018. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks Proc. Natl Acad. Sci 115 E1598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kam JWY, Solbakk A-K, Endestad T, Meling TR and Knighta RT 2018. Lateral prefrontal cortex lesion impairs regulation of internally and externally directed attention Neuroimage 175 91–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Baird B, Smallwood J, Lutz A and Schooler JW 2014. The decoupled mind: mind-wandering disrupts cortical phase-locking to perceptual events J. Cogn. Neurosci 26 2596–607 [DOI] [PubMed] [Google Scholar]
- [162].Fellrath J, Mottaz A, Schnider A, Guggisberg AG and Ptak R 2016. Theta-band functional connectivity in the dorsal fronto-parietal network predicts goal-directed attention Neuropsychologia 92 20–30 [DOI] [PubMed] [Google Scholar]
- [163].Owen LLW, Muntianu TA, Heusser AC, Daly PM, Scangos KW and Manning JR 2020. A Gaussian process model of human electrocorticographic data Cereb. Cortex 30 5333–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Penfield W and Boldrey E 1937. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation Brain 60 389–443 [Google Scholar]
- [165].Solomon EA. et al. Medial temporal lobe functional connectivity predicts stimulation-induced theta power. Nat. Commun. 2018;9:4473. doi: 10.1038/s41467-018-06876-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Parvizi J, Jacques C, Foster BL, Withoft N, Rangarajan V, Weiner KS and Grill-Spector K 2012. Electrical stimulation of human fusiform face-selective regions distorts face perception J. Neurosci 32 14915–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Rangarajan V, Hermes D, Foster BL, Weiner KS, Jacques C, Grill-Spector K and Parvizi J 2014. Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception J. Neurosci 34 12828–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mégevand P, Groppe DM, Goldfinger MS, Hwang ST, Kingsley PB, Davidesco I and Mehta AD 2014. Seeing scenes: topographic visual hallucinations evoked by direct electrical stimulation of the parahippocampal place area J. Neurosci 34 5399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Fox KCR, Shi L, Baek S, Raccah O, Foster BL, Saha S, Margulies DS, Kucyi A and Parvizi J 2020. Intrinsic network architecture predicts the effects elicited by intracranial electrical stimulation of the human brain Nat. Hum. Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Fried I, Wilson CL, MacDonald KA and Behnke EJ 1998. Electric current stimulates laughter Nature 391 650. [DOI] [PubMed] [Google Scholar]
- [171].Caruana F, Gozzo F, Pelliccia V, Cossu M and Avanzini P 2016. Smile and laughter elicited by electrical stimulation of the frontal operculum Neuropsychologia 89 364–70 [DOI] [PubMed] [Google Scholar]
- [172].Mălîia MD, Donos C, Barborica A, Popa I, Ciurea J, Cinatti S and Mîndruţă I 2018. Functional mapping and effective connectivity of the human operculum Cortex 109 303–21 [DOI] [PubMed] [Google Scholar]
- [173].Aminoff EM, Li Y, Pyles JA, Ward MJ, Richardson RM and Ghuman AS 2016. Associative hallucinations result from stimulating left ventromedial temporal cortex Cortex 83 139–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Penfield W and Perot P 1963. The brain’s record of auditory and visual experience: a final summary and discussion Brain 86 595–696 [DOI] [PubMed] [Google Scholar]
- [175].Jacobs J, Lega B and Anderson C 2012. Explaining how brain stimulation can evoke memories J. Cogn. Neurosci 24 553–63 [DOI] [PubMed] [Google Scholar]
- [176].Kucewicz MT et al. 2018. Evidence for verbal memory enhancement with electrical brain stimulation in the lateral temporal cortex Brain 141 971–8 [DOI] [PubMed] [Google Scholar]
- [177].Kucewicz MT. et al. Electrical stimulation modulates high γ activity and human memory performance. eNeuro. 2018;5:1. doi: 10.1523/ENEURO.0369-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Fox KCR and Girn M 2018. Neural correlates of self-generated imagery and cognition throughout the sleep cycle The Oxford Handbook of Spontaneous Thought: Mind-Wandering, Creativity, and Dreaming (New York, NY: Oxford University Press; ) [Google Scholar]
- [179].Fox KCR, Yih J, Raccah O, Pendekanti SL, Limbach LE, Maydan DD and Parvizi J 2018. Changes in subjective experience elicited by direct stimulation of the human orbitofrontal cortex Neurology 91 E1519–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Fox KCR, Andrews-Hanna JR and Christoff K 2016. The neurobiology of self-generated thought from cells to systems: integrating evidence from lesion studies, human intracranial electrophysiology, neurochemistry, and neuroendocrinology Neuroscience 335 134–50 [DOI] [PubMed] [Google Scholar]
- [181].Foster BL and Parvizi J 2017. Direct cortical stimulation of human posteromedial cortex Neurology 88 685–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Parvizi J, Rangarajan V, Shirer WR, Desai N and Greicius MD 2013. The will to persevere induced by electrical stimulation of the human cingulate gyrus Neuron 80 1359–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Blanke O, Ortigue S, Landis T and Seeck M 2002. Stimulating illusory own-body perceptions Nature 419 269–70 [DOI] [PubMed] [Google Scholar]
- [184].Kremmyda O, Kirsch V, Bardins S, Lohr H, Vollmar C, Noachtar S and Dieterich M 2019. Electrical brain stimulation of the parietal lobe impairs the perception of verticality J. Neurol 266 146–8 [DOI] [PubMed] [Google Scholar]
- [185].Popa I, Donos C, Barborica A, Opris I, Mălîia MD, Ene M, Ciurea J and Mîndruţă I 2016. Intrusive thoughts elicited by direct electrical stimulation during stereo-electroencephalography Front. Neurol 7 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Roux FE, Miskin K, Durand JB, Sacko O, Réhault E, Tanova R and Démonet JF 2015. Electrostimulation mapping of comprehension of auditory and visual words Cortex 71 398–408 [DOI] [PubMed] [Google Scholar]
- [187].Wen J, Yu T, Li Y and Li X 2017. Using electrocorticography for presurgical language mapping in epilepsy patients J. Clin. Neurosci 44 320–2 [DOI] [PubMed] [Google Scholar]
- [188].Amengual JL, Vernet M, Adam C and Valero-Cabré A 2017. Local entrainment of oscillatory activity induced by direct brain stimulation in humans Sci. Rep 7 41908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Alagapan S, Lustenberger C, Hadar E, Shin HW and Frhlich F 2019. Low-frequency direct cortical stimulation of left superior frontal gyrus enhances working memory performance Neuroimage 184 697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Alagapan S, Riddle J, Huang WA, Hadar E and Shin HW 2019. Stimulation enhances working memory by modulating phase lag of low-frequency network-targeted, multi-site direct cortical stimulation enhances working memory by modulating phase lag of low-frequency oscillations Cell Rep 29 2590–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Suthana N and Fried I 2014. Deep brain stimulation for enhancement of learning and memory Neuroimage 85 996–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Lee H, Fell J and Axmacher N 2013. Electrical engram: how deep brain stimulation affects memory Trends Cogn. Sci 17 574–84 [DOI] [PubMed] [Google Scholar]
- [193].Ezzyat Y and Rizzuto DS 2018. Direct brain stimulation during episodic memory Curr. Opin. Biomed. Eng 8 78–83 [Google Scholar]
- [194].Jacobs J et al. 2016. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory Neuron 92 983–90 [DOI] [PubMed] [Google Scholar]
- [195].Goyal A et al. 2018. Electrical stimulation in hippocampus and entorhinal cortex impairs spatial and temporal memory J. Neurosci 38 3049–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Jun S, Lee SA, Kim JS, Jeong W and Chung CK 2020. Task-dependent effects of intracranial hippocampal stimulation on human memory and hippocampal theta power Brain Stimul. 13 603–13 [DOI] [PubMed] [Google Scholar]
- [197].Jun S, Kim JS and Chung CK 2019. Direct stimulation of human hippocampus during verbal associative encoding enhances subsequent memory recollection Front. Hum. Neurosci 13 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B and Fried I 2012. Memory enhancement and deep-brain stimulation of the entorhinal area N. Engl. J. Med 366 502–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Titiz AS. et al. Theta-burst microstimulation in the human entorhinal area improves memory specificity. eLife. 2017;6:e29515. doi: 10.7554/eLife.29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Hansen N, Chaieb L, Derner M, Hampel KG, Elger CE, Surges R, Staresina B, Axmacher N and Fell J 2018. Memory encoding-related anterior hippocampal potentials are modulated by deep brain stimulation of the entorhinal area Hippocampus 28 12–7 [DOI] [PubMed] [Google Scholar]
- [201].Koubeissi MZ, Kahriman E, Syed TU, Miller J and Durand DM 2013. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy Ann. Neurol 74 223–31 [DOI] [PubMed] [Google Scholar]
- [202].Natu VS, Lin J-J, Burks A, Arora A, Rugg MD and Lega B 2019. Stimulation of the posterior cingulate cortex impairs episodic memory encoding J. Neurosci 39 7173–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Fell J, Staresina BP, Do ATA, Widman G, Helmstaedter C, Elger CE and Axmacher N 2013. Memory modulation by weak synchronous deep brain stimulation: a pilot study Brain Stimul. 6 270–3 [DOI] [PubMed] [Google Scholar]
- [204].Kim K, Schedlbauer A, Rollo M, Karunakaran S, Ekstrom AD and Tandon N 2018. Network-based brain stimulation selectively impairs spatial retrieval Brain Stimul. 11 213–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Burke JF, Merkow MB, Jacobs J, Kahana MJ and Zaghloul KA 2015. Brain computer interface to enhance episodic memory in human participants Front. Hum. Neurosci 8 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ezzyat Y. et al. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat. Commun. 2018;9:1. doi: 10.1038/s41467-017-02753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Ezzyat Y et al. 2017. Direct brain stimulation modulates encoding states and memory performance in humans Curr. Biol 27 1251–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Mueller SG, Bateman LM, Nei M, Goldman AM and Laxer KD 2019. Brainstem atrophy in focal epilepsy destabilizes brainstem-brain interactions: preliminary findings NeuroImage Clin. 23 101888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Leszczyński M, Barczak A, Kajikawa Y, Ulbert I, Falchier AY, Tal I, Haegens S, Melloni L, Knight RT and Schroeder CE 2020. Dissociation of broadband high-frequency activity and neuronal firing in the macaque neocortex Sci. Adv 6 eabb0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Minxha J, Mamelak AN and Rutishauser U 2018. Surgical and electrophysiological techniques for single-neuron recordings in human epilepsy patients Extracellular Recording Approaches, Neuromethods vol 134, ed Sillitoe RV (Berlin: Springer; ) pp 267–93 [Google Scholar]
- [211].Carlson AA, Rutishauser U and Mamelak AN 2018. Safety and utility of hybrid depth electrodes for seizure localization and single-unit neuronal recording Stereotact. Funct. Neurosurg 96 311–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Vuust P, Ostergaard L, Pallesen KJ, Bailey C and Roepstorff A 2009. Predictive coding of music - brain responses to rhythmic incongruity Cortex 45 80–92 [DOI] [PubMed] [Google Scholar]
- [213].Davis MH, Sohoglu E, Peelle JE and Carlyon RP 2012. Predictive top-down integration of prior knowledge during speech perception J. Neurosci 32 8443–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Taylor JA, Matthews N, Michie PT, Rosa MJ and Garrido MI 2017. Auditory prediction errors as individual biomarkers of schizophrenia NeuroImage Clin. 15 264–73 [DOI] [PMC free article] [PubMed] [Google Scholar]


