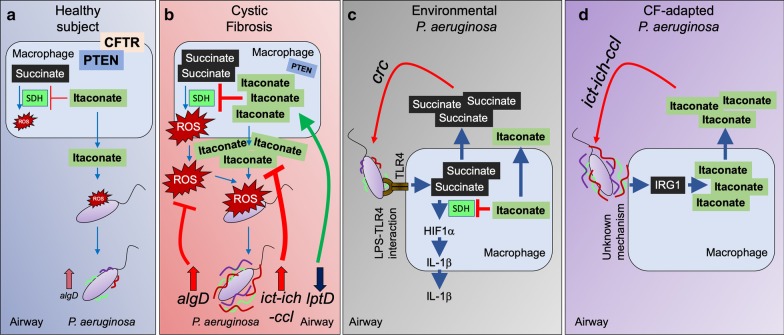
Fig. 3.
Itaconate fuels P. aeruginosa adaptive changes and chronic infection. a In healthy subjects, itaconate is produced during P. aeruginosa infection to control SDH activity, oxidative stress and inflammation. This itaconate levels are tolerated by P. aeruginosa during acute infection. b In CF individuals lacking CFTR-PTEN complex activity, elevated succinate oxidation induces synthesis and release of the anti-oxidant molecule itaconate as a compensatory mechanism. Airway itaconate induces P. aeruginosa outer membrane stress, which induces ict-ich-ccl locus overexpression to degrade itaconate. Itaconate also induces downregulation of lptD, which suppresses surface exposure of LPS. Lack of surface-exposed endotoxin causes bacterial membrane deregulation and permeability, which is compensated by activation of the algT-algD membrane stress response to produce more protective alginate. Through an unknown mechanism, alginate induces more itaconate release by host macrophages, which fuels biofilm production, adaptation and long-term infection. c Environmental P. aeruginosa strains expressing LPS induce the TLR4-succinate-HIF1α-IL-1β axis, inducing release of succinate and regulatory itaconate. Succinate released fuels P. aeruginosa infection through the crc locus during acute infection. d Host-adapted P. aeruginosa isolates, which lack surface LPS and overproduce alginate, induce IRG1 expression and high itaconate production in macrophages. IRG1 induction is mediated by alginate. Itaconate released fuels host-adapted P. aeruginosa through the ict-ich-ccl locus activity. Color lines on P. aeruginosa are extracellular polysaccharides, such as algD-mediated alginate