Figure 2.
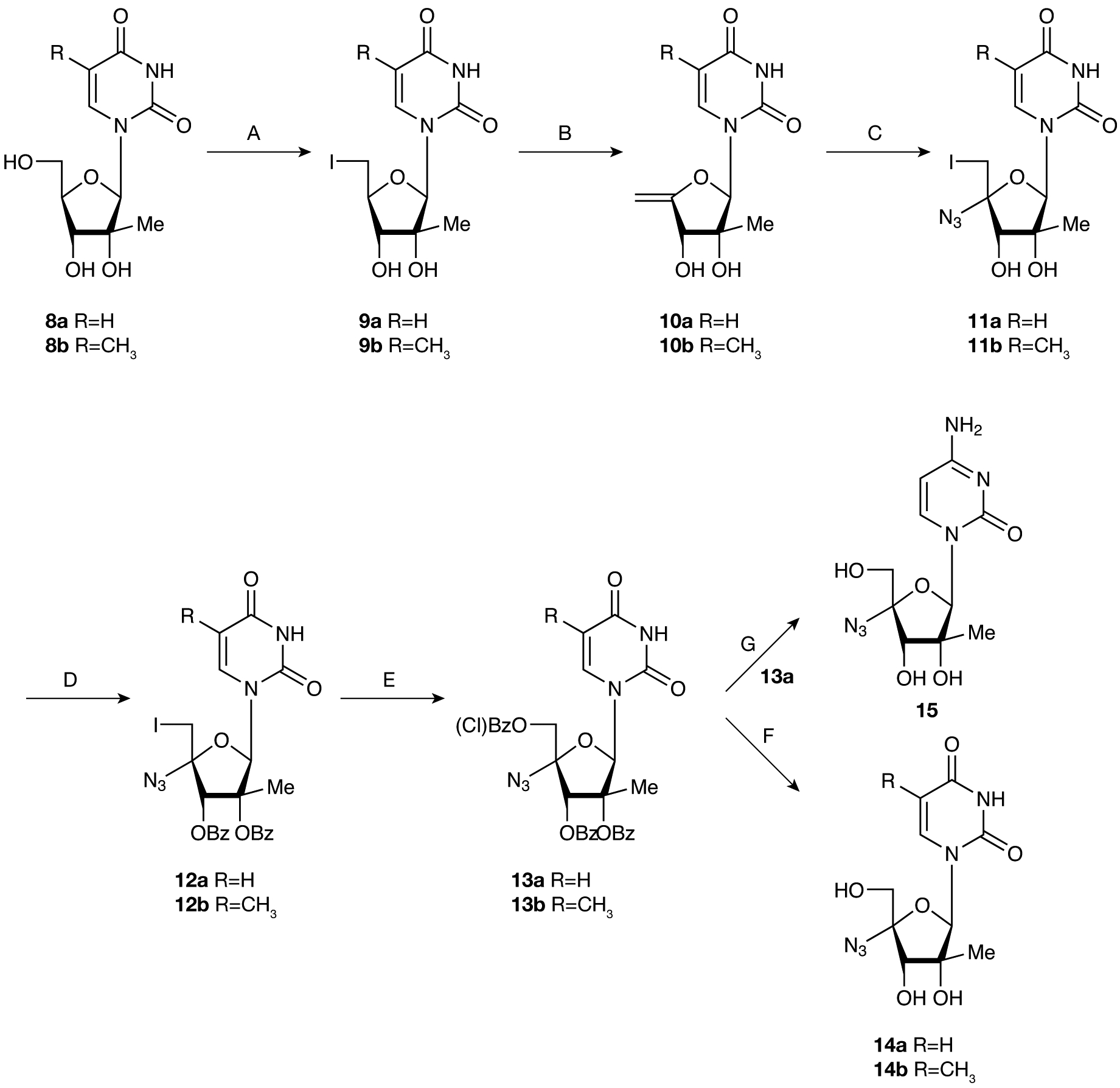
Synthetic route to 2′ C-methyl-4′azido pyrimidine nucleosides
A. I2, Ph3P, imidazole, tetrahydrofuran (THF), rt, (85%; 9a), (81%; 9b). B. NaOCH3, CH3OH, 60°C, (76%; 10a), (78%; 10b). C. ICI, NaN3, THF, (65%; 11a), (61%; 11b). D. BzCl, DMAP, Et3N, CH3CN, rt, (82%; 12a), (85%; 12b). E. meta-Chloroperoxybenzoic acid, CH2Cl2, rt, (62%; 13a), (66%; 13b). F. NH3/MeOH, rt, (86%; 14a), (83%; 14b). G. Step 1: 1,2,4-triazole, Et N, POCl3, CH2Cl2, 0°C to rt. Step 2: NH4OH/CH3CN/MeOH, rt, 42%.
