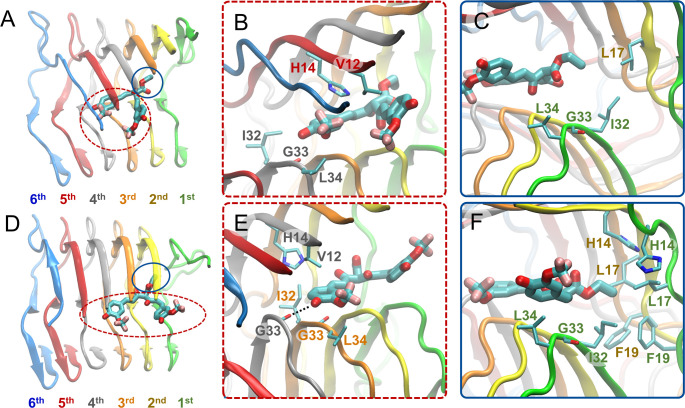
Figure 5.
Molecular graphics images of the lowest energy binding mode 2 for SY12 not leading to partial dissociation (A–C) and leading to partial dissociation (D–F) of the outermost peptide. SY12 is shown in thick licorice representation, the Aβ1–42 fibril and residues are shown in cartoon and thin licorice representation, respectively. (A) Bird’s eye view of SY12 in binding mode nd2. The heads comprising both of the aromatic functional groups and their substituents are circled with a red dotted line. The tail comprising the central R1 group is circled with a blue line. (B) Key interactions between the head groups and Aβ1–42 residues common for all molecules adopting binding mode nd2. (C) Key interactions between the tail group and Aβ1–42 residues common for all molecules adopting binding mode nd2. (D) Bird’s eye view of SY12 adopting binding mode pd2. The anchors are circled with a red dotted line. The breaker is circled with a blue line. (E) Interactions between the anchor groups and Aβ1–42 residues stabilizing SY12 common for all molecules adopting binding mode pd2. (F) Interactions between the breaker group and Aβ1–42 residues common for all molecules adopting binding mode pd2.