Figure 10:
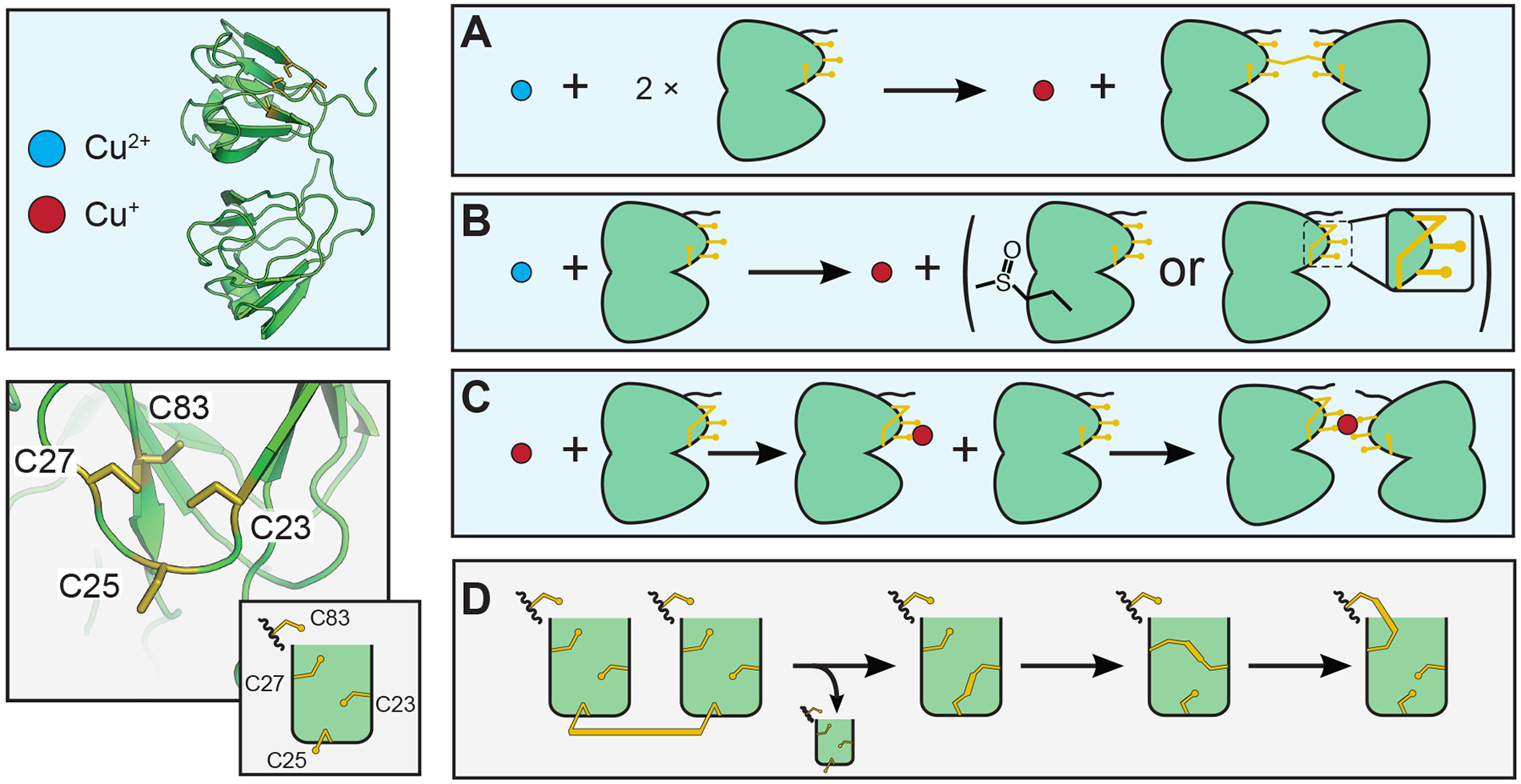
Potential interactions of γS-crystallin involving copper and disulfide bond (shown in gold) transfers. (A) Reduction of Cu(II) to Cu(I) and oxidation of two γS-crystallins resulting in an intermolecular disulfide. (B) Cu(II) is reduced to Cu(I) while γS is oxidized. Oxidation may cause methionine sulfoxide, cysteine sulfenic acid, or intramolecular disulfide bond formation. (C) Cu(I) binding to γS-crystallin may lead to the formation of a metal-bridged dimer. (D) Disulfide bond transfer between γS-crystallins most likely involves C25. Although the exact transfer pathway is not yet known, it is clear that C83 is involved in the final intramolecular disulfide. So far the partner cysteine has not been definitively identified; however, barring a large structural rearrangement, C23 and/or C27 must be involved in any intermediary isomerization.
