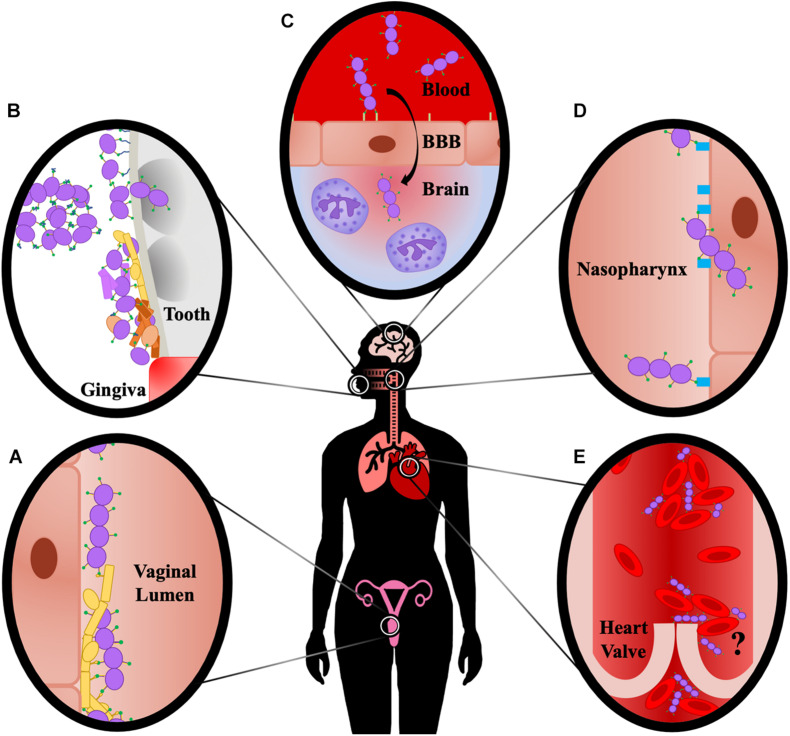
FIGURE 3.
Summary of Site-Specific AgI/II Interactions. (A) AgI/II proteins promote direct interactions with vaginal epithelial cells, and interact with Als3 protein of C. albicans, promoting fungal and streptococcal adherence to the vaginal epithelium. (B) AgI/II proteins bind fluid-phase SAG/gp340, resulting in aggregation and clearance through mechanisms such as swallowing, as well as to immobilized SAG/gp340 on the tooth pellicle, facilitating adherence and colonization. AgI/II proteins mediate direct adherence to tooth components and invasion into dentinal tubules, resulting in caries formation, and also facilitate colonization and polymicrobial biofilm formation with cariogenic pathogens, as well as with pathogens associated with periodontitis. (C) AgI/II proteins interact with vimentin on the surface of brain endothelium, and also induce chemokine signaling and neutrophil chemotaxis characteristic of meningitis. (D) AgI/II proteins interact with β1 integrin on the nasopharynx epithelium, resulting in adherence and invasion of the cells and consequently increased bacterial burden in the nasopharynx and lungs. (E) AgI/II proteins promote aggregation of blood-borne bacteria, as well as attachment to and aggregation of platelets; however, there is currently insufficient evidence to show this promotes increased vegetation formation on heart valves that is characteristic of infective endocarditis, as indicated by the question mark.