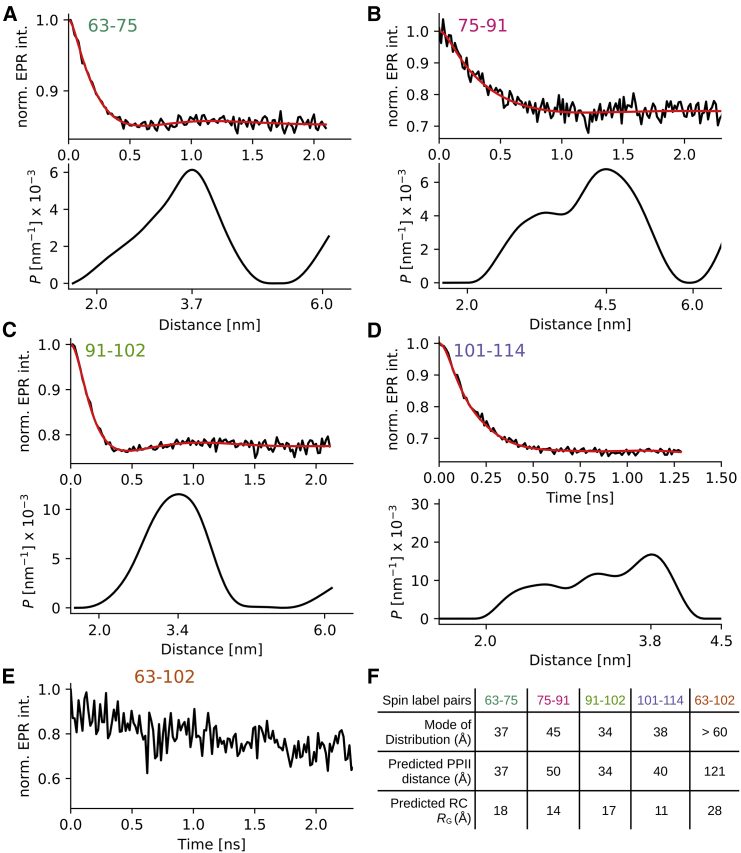
Figure 2.
Distance distributions in the C-terminus of HTTex1 fusion protein measured via EPR DEER spectroscopy. (A–D) Top panels: baseline corrected DEER data (black) and fit using Tikhonov regularization is shown (red). Bottom panels: distance distribution PDEER corresponding to fit is shown. The mode of the distribution is indicated using a tick mark. (E) Same as (A–D) but because the distance between 63 and 102 was above the detection limit, only the raw data without baseline subtraction and fit are shown. Raw data of the other distances are shown in Fig. S1. (F) Table listing mode of individual distances distributions is shown. For comparison, the theoretical distances for a polyP II helix assuming an increase of 3.1 Å per residue and the theoretical radius of hydration (RH) for a random coil, are given (RH was calculated using the formula by Marsh and Forman-Kay (44)). Both distances spanning the polyP regions (P11 and P10) correspond nicely to a PPII distance. The distances between residues 75–91 and 101–114 are significantly shorter than a PPII helix and longer than that expected for a random coil structure. To see this figure in color, go online.