Figure 8.
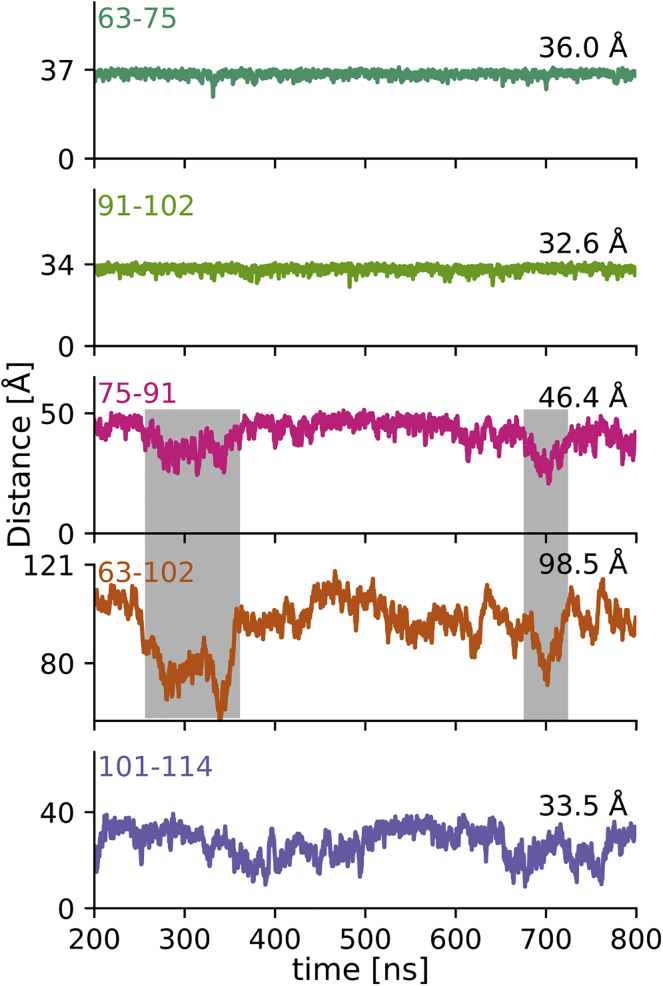
The L17 region is responsible for the overall kinks of the PRD. Change in Cα-Cα distances (as defined in Fig. 1) over time. The upper y axis tick indicates an ideal polyproline II helical distance (see Fig. 2 F); the mode of simulated Cα-Cα distance distribution is indicated on the right. All distance variations are plotted with comparable y axis scaling. Both polyP stretches stay in an extended PPII conformation for most of the simulation. The L17 region is a less ideal PPII helix. Its deviations from an extended PPII conformation induce an overall kink in the PRD as illustrated by the correlated shortening of distance 63–102 (gray boxes). The C12 region is the most flexible and, although still relatively extended, deviates from a PPII helix most of the time. To see this figure in color, go online.