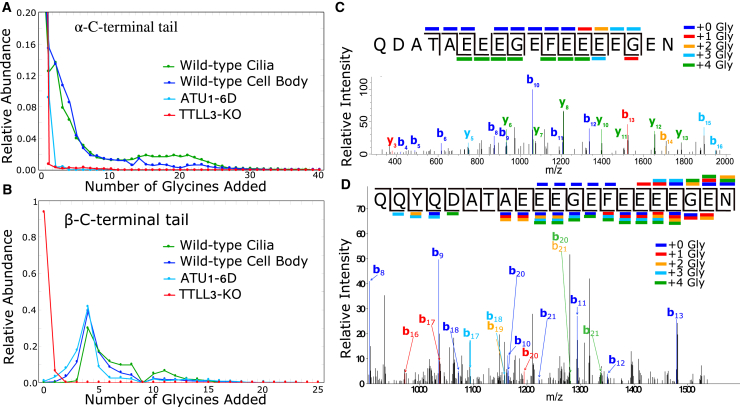
Figure 3.
Mass spectrometry characterization of the polyglyclation patterns on differentially modified tubulin samples. For the wild-type samples, we purified tubulin from both ciliary and cell body fractions. (A and B) We measured the total ion current corresponding to trypsin (α) or chymotrypsin (β) C-terminal peptides, which include the disordered CTTs. We found a peak in the β-tubulin tail sample of four additional glycines. We mapped the glycyine additions of this parent peptide using MS/MS (41,42). (C) An example MS/MS scan shows fragmentation consistent with a single glycine on each of the four terminal glutamates. (D) Shown is a different MS/MS scan containing fragments corresponding to a wide rage of glycine localizations. Fragmentation of the modification itself is thought to contribute to the diversity of y ions. We did not observe b ions consistent with modifications upstream of the four terminal glutamates. To see this figure in color, go online.