Abstract
Serotonin syndrome (SS) is a drug-induced clinical syndrome, characterised by a triad of cognitive impairment, autonomic hyperactivity and neuromuscular abnormalities. Hypertension, one of the common autonomic manifestations in SS, may lead to lead to several life-threatening conditions. Herein, we report a case of SS who had posterior reversible encephalopathy syndrome (PRES) because of high blood pressure.
A young male with a 5-month history of chronic tension-type headache and depression had been receiving amitriptyline and paroxetine. Increment of paroxetine led to the development of various new clinical features, fulfilling the Hunter criteria of SS. MRI brain revealed high-signal intensity lesions on T2 fluid-attenuated inversion recovery, and T2-weighted imaging in the posterior regions of the occipital, parietal, temporal and cerebellum lobes, suggestive of PRES. The patient responded to cyproheptadine. Autonomic hyperactivity, due to SS, is the most likely explanation of this association.
Keywords: neuro ITU, drugs misuse (including addiction), unwanted effects / adverse reactions
Background
Serotonin syndrome (SS) is a drug-induced clinical syndrome, characterised by a triad of cognitive impairment, autonomic hyperactivity and neuromuscular abnormalities. Cognitive impairment may range from mild irritability to deep coma. Neuromuscular abnormalities of SS encompass a wide range of clinical symptoms and physical signs.1 Autonomic manifestations include mild nausea, vomiting, diarrhoea, dizziness, tachycardia, hypertension, mydriasis, hyperactive bowel sounds, diaphoresis, fever and sexual dysfunctions. Autonomic manifestations may be the presenting complaint in a subset of patients with SS. Hypertension, one of the common clinical features associated with SS, is typically mild to moderate in severity, not requiring any specific therapy.1 However, severe dysautonomia may occur in some patients, leading to hypertensive crisis, posterior reversible encephalopathy syndrome (PRES), myocardial infarction and even death.2–4 We noted a case of SS with PRES. To date, only one such case has been reported in the literature.4
Case presentation
A 37-year-old man had a history of chronic tension-type headache and depression for 4–5 months. He had been taking amitriptyline (50 mg daily) for the last 4 weeks, and paroxetine (12.5 mg daily) had been added as there was not much improvement in the symptoms. The patient was instructed to increase the dose of paroxetine to 25 mg daily after 7 days. Two days after the increment of paroxetine, the patient experienced generalised bodily pain accompanied by irritability, dizziness and transient visual obscurations. However, he continued to take both drugs as usual, and the symptoms gradually worsened. Two days later, he noted unsteadiness in the gait and he was not able to walk in a straight line. It was followed by tremulousness all over the body, especially in the limbs, and he could not perform any manual works smoothly. The symptoms gradually worsened, and later, he developed slurring of speech, fever and diaphoresis.
The patient was brought to the emergency department, and on arrival, he was agitated and diaphoretic. He could not stand properly and needed assistance to stand and walk. Tremulousness was obvious and myoclonic jerks were noted in the limbs. He spoke only incomprehensible sounds. General physical examination on arrival revealed high temperature (100.8°F), tachycardia (110 beats/min), hypertension (176/94 mm Hg) and increased respiratory rate (26 breaths/min). He had no preceding history of hypertension and any other major illness.
Systemic examination demonstrated a clear chest, normal heart sounds and hyperactive bowel sounds. On neurological examination, the patient was alert, but agitated. A detailed mental examination was not done as his speech was incomprehensible. An examination of the cranial nerves, including optic disc, did not show any abnormalities. Detailed motor examinations revealed coarse postural tremor, generalised hypertonia, brisk deep tendon reflex, inducible clonus at the ankles and knees, extensor plantar response, and incoordination in both upper and lower limbs. The patient was suspected of having SS, and the clinical features fulfilled the Hunter criteria of SS.5 As SS is considered as a medical emergency, cyproheptadine and clonazepam had been started immediately, and amitriptyline and paroxetine were discontinued. At the same time, the patient was subjected to various investigations.
Investigations
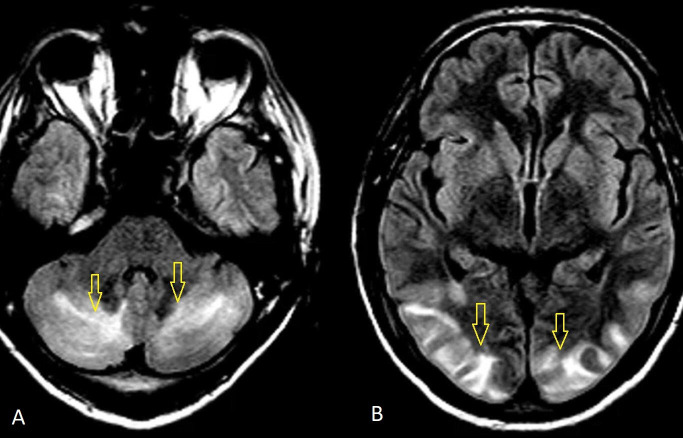
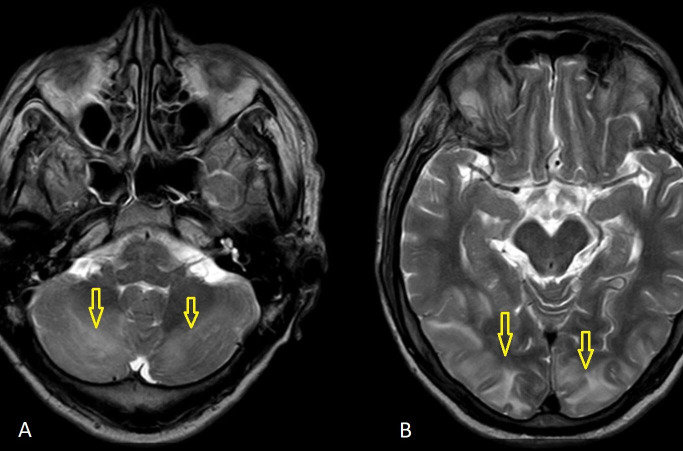
All biochemical and haematological parameters were normal, except mild leucocytosis (white blood cell count 12 900 mm3). An ECG revealed sinus tachycardia. MRI brain was performed on the second day of admission and it revealed high-signal intensity lesions on T2 fluid-attenuated inversion recovery (FLAIR) (figure 1A, B), and T2-weighted imaging (T2W) (figure 2) in the posterior regions of the occipital, parietal, temporal and cerebellum lobes. MRI suggested a possibility of PRES. Cerebrospinal fluid (CSF) analysis and electroencephalography showed no abnormality.
Figure 1.
(A, B) MRI brain: T2 fluid-attenuated inversion recovery sequences showing hyperintense signals in the occipital, parietal and cerebellum lobes (arrows).
Figure 2.
(A, B) MRI brain: T2-weighted imaging sequences showing hyperintense signals in the occipital, parietal and cerebellum lobes (arrows).
Differential diagnosis
A diagnosis of PRES with SS was obvious. However, a possibility of viral encephalitis, acute disseminated encephalomyelitis and posterior circular stroke were also considered. However, characteristic imaging findings, biochemical parameters and CSF analysis ruled out other possibilities. Moreover, a prompt response to cyproheptadine (for SS) further excludes other secondary possibilities, as a resolution of other pathologies could not be so immediate.
Treatment
Twelve milligrams of loading dose of cyproheptadine was administered immediately along with supportive care. It was followed by 2 mg every 2 hours. Clonazepam (0.5 mg two times daily) was also administered.
Outcome and follow-up
Approximately 8 hours after the therapy, the patient was not agitated and his speech was comprehensible. Heart rate (88 beats/min), blood pressure (130/84 mm Hg) and temperature (98.8°F) gradually returned to normal levels in 24 hours. On the second day, there were no diaphoresis, tremulousness, myoclonus and clonus. Hypertonia, hyperreflexia and incoordination improved markedly, and he was able to walk unassisted. MRI brain was done on the second day. Most of the symptoms either disappeared or significantly improved when we got the MRI suggesting PRES. The patient was closely monitored for the fluctuation of blood pressure, and it remained within the normal range. The patient was re-examined for visual disturbances, a common and specific symptom of PRES of the parietal–occipital pattern. The patient confirmed the presence of transient visual obscuration, but visual field examination did not reveal any abnormalities. The dosage of cyproheptadine was reduced to 8 mg three times a day after 36 hours. While hypertonia and hyperreflexia disappeared in 2–3 days, incoordination resolved in 5–6 days. Cyproheptadine was gradually tapered and discontinued after 2 weeks. There was no recurrence of symptoms, including hypertension, during the 9-month follow-up. A follow-up MRI of the brain performed after 2 months revealed no abnormalities.
Discussion
With inducible clonus, agitation, diaphoresis, fever, tremor, hypertonia and hyperreflexia, this patient fulfilled the Hunter diagnostic criteria of SS. The temporal relation between the administration of cyproheptadine and the resolution of clinical symptoms further strengthens the diagnosis of SS. Normalisation of hypertension by cyproheptadine suggests that blood pressure was part of SS.
PRES is a clinical syndrome that has three key components: acute neurological features, specific neuroimaging findings and an obvious risk factor. The typical clinical features of PRES include headache, nausea/vomiting, altered mental status, seizure, visual disturbances and cerebellar ataxia. On neuroimaging, PRES is typically characterised by vasogenic oedema without infarction, involving predominantly bilateral subcortical areas of the parietal and occipital lobes. It typically presents in patients with acute hypertensive disorders. However, it has also been described after immunosuppressive therapy, infection, sepsis, autoimmune diseases, renal disorders and hypercalcemia.6 Clinical features, neuroimaging abnormalities and associated hypertension were consistent with a diagnosis of typical PRES in this patient. However, PRES was not the working diagnosis, and we treated the patient on the line of SS. The underlying PRES was revealed (on MRI brain) when most of the symptoms either disappeared or reduced significantly with the treatment of SS. So, we hypothesise that PRES was part of SS which occurred due to autonomic hyperactivity. There are marked overlaps between the clinical spectrum of SS and PRES. So, it is very difficult to recognise the symptoms that were specifically related to PRES in this patient. Visual disturbance and incoordination may be predominantly because of PRES.
The first case of PRES was described by Hinchey et al in 1996.7 Since then, PRES has been increasingly recognised. Although the incidence of PRES is not known in the general population, a few studies have described its incidence in a selected group of patients. PRES has been noted in 2.7%–25% of patients after bone marrow transplantation, 0.84% of patients with end-stage kidney disease and about 0.69% of patients with systemic lupus erythematosus.8 More than 90% patients with eclampsia may have PRES.9 In a recent review, Largeau and colleagues noted 42 cases of PRES due to poisoning or toxin ingestion.10 So, PRES is not rare in clinical practice. A wide range of triggers have been identified for PRES. Acute hypertension is the most common trigger for PRES, and several causes of acute hypertension, leading to PRES, have been reported in the literature.6 Autonomic dysautonomia may cause severe hypertension, and it may trigger PRES. There are several case reports of PRES in patients with Guillain-Barré syndrome (GBS). Hypertension due to dysautonomia is the most likely explanation for this association.11 However, a few patients with GBS may have PRES because of intravenous immunoglobulin therapy.
Just like GBS, patients with SS have autonomic hyperactivity. Hypertension is one of the common manifestations of SS, and it may be noted in 50%–75% of the patients with SS.12 13 Ott et al performed a systemic review on SS to find the various aspects of hypertension in SS.3 About 13% of the patients (49 out of 374) had severe hypertension (systolic blood pressure >175 mm Hg). Severe hypertension, associated with SS, may cause several other complications, including hypertensive crisis, myocardial infarction and death. To the best of our literature search, there is only one case report of PRES in association with SS. However, the previous case of PRES in SS had multiple risk factors for the development of PRES. The patient had a history of hypertension, end-stage renal disease and a recent history of dialysis.4 So, in addition to SS, there were multiple contributing factors for the development of PRES. In our case, SS was the only factor leading to PRES.
There are two proposed hypotheses for the pathogenesis of PRES: impaired cerebral autoregulation and endothelial dysfunctions. Impaired cerebral autoregulation is seen in the setting of acute hypertension, and it leads to hyperperfusion, breaking the blood–brain barrier and interstitial oedema.6 This is the most likely explanation in our patient. The same mechanisms have been suggested for the development of PRES in patients with GBS with dysautonomia.11
The treatment of PRES is largely supportive, including control of raised blood pressure. The management of SS includes discontinuation of serotonergic agents, supportive care and 5-HT2A antagonist (cyproheptadine).1 SS typically resolves within 24 hours after the initiation of therapy. Hypertension usually resolves with other symptoms and patients do not require specific antihypertensive drugs.1 In Ott et al’s review, only 10 patients with severe hypertension (out of 49 patients) received specific anti-hypertensive therapy.3 Other patients responded to cyproheptadine and other supportive care (including benzodiazepines). As autonomic activities in SS often fluctuate markedly, short-acting anti-hypertensive agents (esmolol or nitroprusside) are recommended for the management of hypertension.1 However, conventional antihypertensive may not work in patients with SS. A higher dose of cyproheptadine should be tried in such patients. A response to cyproheptadine suggests that hypertension is because of serotonin toxicity rather than a hyperadrenergic state.3
SS is a highly underdiagnosed clinical condition, and underdiagnosis may be partly because of physicians’ unawareness about SS and partly because of protean manifestations of SS.1 14 Although the Hunter criteria include only a few symptoms and signs, SS encompasses a myriad of clinical symptoms and physical signs that may mimic a variety of medical conditions.9 The co-occurrence of PRES and SS is unusual and can easily escape recognition, especially if the patient is not subjected to neuroimaging (MRI brain). MRI brain is not routinely advised in patients with SS.1 Sharing this experience may increase awareness about the heterogeneity of SS. Recent observations have suggested that SS may occur in all clinical settings. Buckley et al suggest that all doctors need to be aware of various aspects of SS.15 The central feature of the Hunter criteria is clonus (spontaneous or induced). The examination for clonus is often omitted in clinical practice, including ICU setting.14 With this case report, we highlight the importance of examination for clonus in the emergency setting. We also suggest the need for MRI brain in patients with SS having marked autonomic hyperactivities.
Limitation
This case fulfilled the diagnostic criteria of SS and MRI brain abnormalities were typical of PRES. However, we cannot rule out the possibility of any other cause for the recent clinical picture as full investigations were not done. Moreover, the interrelation of SS with PRES is purely circumstantial. A possibility of two different disease entities cannot be ruled out.
Conclusion
The clinical features of SS include a wide range of symptoms. Hypertension due to autonomic hyperactivity in SS may produce PRES.
Patient’s perspective.
I was not aware that headache medicines could do such problems. I would control my headaches with life style modifications only.
Learning points.
Serotonin syndrome (SS) encompasses a wide range of clinical symptoms and physical signs.
Hypertension due to autonomic hyperactivity in SS may produce posterior reversible encephalopathy syndrome.
Hypertension of SS may respond to a 5-HT2A antagonist.
Footnotes
Contributors: SP, CR and RK were involved in the conception and design. SP and RK were involved in the acquisition of data. SP was involved in the manuscript preparation. CR and RK were involved in revising the draft for intellectual content. SP, CR and RK were involved in the final approval of the completed manuscript. SP was the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005;352:1112–20. 10.1056/NEJMra041867 [DOI] [PubMed] [Google Scholar]
- 2.de Castro Julve M, Miralles Albors P, Ortonobes Roig S, et al. . Hypertensive crisis following the administration of tedizolid: possible serotonin syndrome. Eur J Hosp Pharm 2020;27:52–4. 10.1136/ejhpharm-2018-001752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott M, Mannchen JK, Jamshidi F, et al. . Management of severe arterial hypertension associated with serotonin syndrome: a case report analysis based on systematic review techniques. Ther Adv Psychopharmacol 2019;9:204512531881881. 10.1177/2045125318818814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik MT, Majeed MF, Zand R. Serotonin syndrome presenting as a posterior reversible encephalopathy syndrome. Case Rep Neurol 2020;12:63–8. 10.1159/000505907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunkley EJC, Isbister GK, Sibbritt D, et al. . The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003;96:635–42. 10.1093/qjmed/hcg109 [DOI] [PubMed] [Google Scholar]
- 6.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914–25. 10.1016/S1474-4422(15)00111-8 [DOI] [PubMed] [Google Scholar]
- 7.Hinchey J, Chaves C, Appignani B, et al. . A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500. 10.1056/NEJM199602223340803 [DOI] [PubMed] [Google Scholar]
- 8.Hinduja A. Posterior reversible encephalopathy syndrome: clinical features and outcome. Front Neurol 2020;11:71. 10.3389/fneur.2020.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg RK, Kumar N, Malhotra HS. Posterior reversible encephalopathy syndrome in eclampsia. Neurol India 2018;66:1316. 10.4103/0028-3886.241364 [DOI] [PubMed] [Google Scholar]
- 10.Largeau B, Boels D, Victorri-Vigneau C, et al. . Posterior reversible encephalopathy syndrome in clinical toxicology: a systematic review of published case reports. Front Neurol 2019;10:1420. 10.3389/fneur.2019.01420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi S, Prentice D, van Heerden J, et al. . Guillain-Barré syndrome and posterior reversible leukoencephalopathy syndrome: a rare association. BMJ Case Rep 2020;13:e234184 10.1136/bcr-2019-234184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash S, Rathore C, Rana KK, et al. . Refining the clinical features of serotonin syndrome: a prospective observational study of 45 patients. Ann Indian Acad Neurol 2019;22:52–60. 10.4103/aian.AIAN_344_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Werneke U, Jamshidi F, Taylor DM, et al. . Conundrums in neurology: diagnosing serotonin syndrome—a meta-analysis of cases. BMC Neurol 2016;16:97. 10.1186/s12883-016-0616-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakash S, Rathore C, Rana K. Knowledge, attitude, and practice (KAP) study on serotonin syndrome among nNeuro physicians. Ann Indian Acad Neurol 2020. 10.4103/aian.AIAN_603_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ 2014;348:g1626. 10.1136/bmj.g1626 [DOI] [PubMed] [Google Scholar]