Abstract
Introduction
The previously published SYNTAX III REVOLUTION trial demonstrated that clinical decision-making between coronary artery bypass graft (CABG) and percutaneous coronary intervention based on coronary CT angiography (CCTA) had a very high agreement with the treatment decision derived from invasive coronary angiography (ICA). The study objective of the FASTTRACK CABG is to assess the feasibility of CCTA and fractional flow reserve derived from CTA (FFRCT) to replace ICA as a surgical guidance method for planning and execution of CABG in patients with three-vessel disease with or without left main disease.
Methods and analysis
The FASTTRACK CABG is an investigator-initiated single-arm, multicentre, prospective, proof-of-concept and first-in-man study with feasibility and safety analysis. Surgical revascularisation strategy and treatment planning will be solely based on CCTA and FFRCT without knowledge of the anatomy defined by ICA. Clinical follow-up visit including CCTA will be performed 30 days after CABG in order to assess graft patency and adequacy of the revascularisation with respect to the surgical planning based on non-invasive imaging (CCTA) with functional assessment (FFRCT) and compared with ICA. Primary feasibility endpoint is CABG planning and execution solely based on CCTA and FFRCT in 114 patients. Primary safety endpoint based on 30 day CCTA is graft assessment and topographical adequacy of the revascularisation procedure. Automatic non-invasive assessment of functional coronary anatomy complexity is also evaluated with FFRCT for functional Synergy Between percutaneous coronary intervention With Taxus and Cardiac Surgery Score assessment on CCTA. CCTA with FFRCT might provide better anatomical and functional analysis of the coronary circulation leading to appropriate anatomical and functional revascularisation, and thereby contributing to a better outcome.
Ethics and dissemination
Each patient has to provide written informed consent as approved by the ethical committee of the respective clinical site. Results will be submitted for publication in peer-reviewed journals and will be disseminated at scientific conferences.
Trial registration number
Keywords: cardiology, coronary heart disease, cardiovascular imaging, cardiac surgery, cardiothoracic surgery, ischaemic heart disease
Strengths and limitations of this study.
The FASTTRACK coronary artery bypass graft (CABG) study evaluates the feasibility and safety of planning and execution of CABG solely based on CT angiography (CCTA) combined with fractional flow reserve derived from CTA (FFRCT) without knowledge of the anatomy defined by invasive coronary angiography.
At 30 days after CABG, postsurgical CCTA is planned to evaluate the patency of bypass grafts and topographical adequacy of the revascularisation procedure.
CCTA with FFRCT might provide better anatomical and functional analysis of the coronary circulation leading to appropriate anatomical and functional revascularisation, and thereby contributing to a better outcome.
The FASTTRACK CABG is a first-in-man and proof-of-concept study with feasibility and safety analysis designed to provide preliminary observations and generate hypotheses.
Based on the previous literature, the study is considered positive if the lower boundary of confidence intervals for the feasibility rate in percentage exceeds at least 75%, which approximately corresponds to a feasibility rate of 84%.
Introduction
Since the introduction of coronary artery bypass grafting (CABG), surgical revascularisation has evolved as the treatment of choice for patients with complex coronary artery disease.1 The extent and complexity of coronary artery disease objectively assessed by the anatomical Synergy Between percutaneous coronary intervention (PCI) With Taxus and Cardiac Surgery (SYNTAX) Score combined with clinical characteristics and comorbidities play a decisive role when deciding on the revascularisation strategy.2–5 As a pilot survey of surgeons, the SYNTAX III REVOLUTION trial has demonstrated that clinical decision-making between CABG and PCI based on coronary CT angiography (CCTA) performed with the high-definition GE Healthcare Revolution CT scanner has a very high agreement (93% and Cohen’s kappa 0.82) with the treatment decision derived from invasive coronary angiography (ICA) in patients with three-vessel disease with or without left main disease.6
CCTA has emerged as a non-invasive tool able to provide an accurate assessment of coronary artery disease even in complex lesion subsets.7–10 Physiological assessment with fractional flow reserve derived from CT angiography (FFRCT) has been shown to be accurate in patients with multivessel disease.11 Of note, the National Institute for Health and Care Excellence in UK has published a guidance on February 2017 recommending coronary CTA using HeartFlow FFRCT in patients with recent onset chest pain prior to or in replacement of diagnostic test such as echocardiography, stress echocardiography, myocardial perfusion imaging, stress MRI, CT calcium scoring and ICA.12 By adopting this diagnostic approach, the National Health Service in England estimates that they will save a minimum of £9.1 million by 2022. In the European Society of Cardiology guidelines for the diagnosis and management of chronic coronary syndrome (CCS), using CCTA for the patients with extensive coronary calcification, irregular heart rate, significant obesity or inability to cooperate with breath-hold commands is described as the class III (Is not recommended).13 The limitation of FFRCT is almost similar to CCTA. However, FFRCT provided high and superior diagnostic performance compared with CCTA alone in patients and vessels with a high Agaston score.14 In addition, the subanalysis of the PACIFIC (Prospective Comparison of Cardiac PET/CT, SPECT/CT Perfusion Imaging and CT Coronary Angiography With Invasive Coronary Angiography (PACIFIC) study) trial demonstrated that FFRCT showed significantly higher diagnostic performance than the others non-invasive tests (CCTA, single photon emission CT, and positron emission tomography) to evaluate ischaemic heart disease referenced by invasive FFR.15
European and American guidelines recommend a heart team approach (cardiac surgeon, interventional cardiologist and cardiologist) for the decision-making process between CABG and PCI (class I recommendation).16 17 The surgeons were part of the multidisciplinary heart team that included interventional cardiologists and radiologists. During the conduct of the SYNTAX III Revolution trial, careful examination and assessment of atherosclerotic plaques, including calcium burden and interpretation of lesion-specific ischaemia by FFRCT, prompted the surgeons to raise the hypothesis that CCTA coupled with FFRCT might provide sufficient information, even superior to ICA in planning CABG.6 However, the feasibility and safety of this approach remain to be assessed and proved. The aim of the present study is to assess the feasibility of CCTA and FFRCT to replace ICA as a surgical guidance method for planning and execution of CABG in patients with three-vessel disease with or without left main disease.
Methods and analysis
Study purpose
The study objective is to assess the capability of CCTA to replace ICA as a guidance for surgeons in the execution of CABG in patients with established three-vessel disease with or without left main disease.
Study design
The FASTTRACK CABG study is an investigator-initiated single-arm, multicentre, prospective, proof-of-concept, and first-in-man study in patients with three-vessel disease with or without left main involvement referred to CABG treatment. Surgical revascularisation strategy and treatment planning will be solely based on CCTA and FFRCT without knowledge of the anatomy defined by ICA. The ICA will be used in the treatment decision-making process (eg, PCI/CABG) by the regular and ‘Conventional Heart Team’ which will not be involved in the ‘CCTA Planning and Operating Heart Team’ or in the actual surgical treatment (figure 1). One clinical follow-up visit including CCTA will be performed at 30 days after CABG in order to assess graft patency and topographical adequacy of the revascularisation with respect to the surgical planning based on non-invasive imaging. Clinical data will be adjudicated by an independent Clinical Event Committee. Ongoing safety monitoring will be performed by a Data Safety Monitoring Board. The flowchart of this study is shown in figure 1. Each patient has to provide written informed consent as approved by the Ethical Committee of the respective clinical site.
Figure 1.
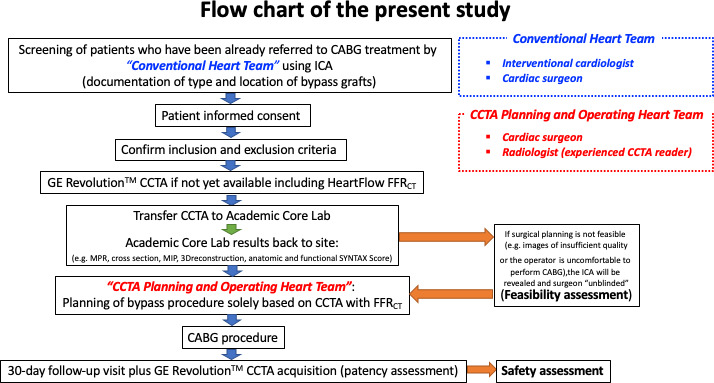
Flowchart. CABG, coronary artery bypass graft; CCTA, coronary CT angiography; FFRCT, fractional flow reserve derived from CT angiography; ICA, invasive coronary angiography; MIP, maximum intensity projection; MPR, multiplanar reconstruction; SYNTAX Score, Synergy Between percutaneous coronary intervention With Taxus and Cardiac Surgery Score.
Statistical consideration
This is a single-arm safety and feasibility study designed to provide preliminary observations and generate hypotheses. No formal statistical sample size calculation has been performed, however, the feasibility evaluation in 100 patients is aimed. In the SYNTAX III Revolution trial, FFRCT was available in 196/223 patients (88%). Taking into consideration a combined potential attrition of CCTA and FFRCT of 12%, 114 patients will be included.6 In preparation of this first-in-man study, five cardiac surgeons initially involved in the SYNTAX III Revolution trial were asked to review retrospectively the randomised cases allocated to surgery and to decide on an individual basis whether the surgical planning of the cases was feasible based on the sole evaluation of CCTA and FFRCT.18 In this survey, cardiac surgeons agreed in 84% of cases to plan and perform CABG solely guided by the CCTA information including FFRCT. It means that in 16% of the cases, the surgeon required the viewing of ICA. Based on the previous literature, the study is considered positive if the lower boundary of confidence intervals for the feasibility rate in percentage exceeds at least 75%, which approximately corresponds to a feasibility rate of 84%.19
Primary endpoints
Primary feasibility endpoint is feasibility expressed in percentage of CABG planning and execution solely based on CCTA in 114 candidates for CABG (ie, the ‘CCTA Planning and Operating Heart Team’ and the operator being blind for the ICA) (percentage/rate). Primary safety endpoint based on 30 day CCTA is the rate of graft stenosis (≥50% diameter stenosis (DS) – 99% DS) or occlusion (100% DS) either at the ostium, in the shaft or at the level of the sequential anastomosis or at the distal anastomosis of each individual graft. The primary safety endpoint (graft patency) will be evaluated per type of graft with respect to historical series of angiographic controls performed by ICA or CCTA at 30 days (online supplemental file).
bmjopen-2020-038152supp001.pdf (2.2MB, pdf)
Secondary endpoints
Secondary feasibility endpoints include the following: (1) CCTA planned revascularisation (anatomic and functional) versus the actually executed CABG treatment (topographical adequacy of revascularisation) and graft patency of the graft (ie,<50%, ≥50%, ≥75% or 100% stenosis); (2) CCTA planned revascularisation versus planned revascularisation based on ICA (by non-operating surgeons involved in the ‘Conventional Heart Team’ based on assessment of ICA performed either in the investigational centre or referred from an affiliate ‘external centre’); (3) planned revascularisation based on ICA versus the actually executed revascularisation by the investigational centre.
Secondary safety endpoints include the following: (1) major adverse cardiac and cardiovascular events at hospital discharge and at 30 days: (a) death (all deaths, cardiac death, vascular death, cardiovascular death); (b) stroke (modified Rankin Scale≥1)20; (c) myocardial infarction (MI) according to the Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) study definition21; (d) all revascularisations (PCI or re-CABG), including target vessel and non-target vessel, if applicable; (2) need for angiography after CABG procedure prior to the 30 day follow-up visit due to emergent clinical status; (3) bleeding (Bleeding Academic Research Consortium-4); (4) procedural complications (online supplemental file); (5) other serious adverse events (SAEs); (6) anatomical SYNTAX Score calculation based on non-invasive GE Healthcare Revolution CT (visual by site and by Academic Core Laboratory); (7) functional SYNTAX Score by FFRCT by Academic Core Laboratory; (8) residual anatomic and functional post-CABG SYNTAX Score based on 30 day CCTA by Academic Core Laboratory.22
Patient population
One hundred fourteen (114) patients referred for CABG will be enrolled in three study sites in Europe and will constitute the study population for the feasibility and safety assessment. The trial was originally intended to take place in the four sites including the University Hospital of Zurich. However, the trial is no longer going ahead at that site due to unforeseen circumstances. By protocol, all patients enrolled will be already assigned and referred to CABG treatment by the ‘Conventional Heart Team’. All sites will be using high-definition GE Healthcare Revolution CT and HeartFlow FFRCT. All diagnostic tools and surgical devices used in this study are CE marked, that is, are not investigational and are used within intended use.
Inclusion criteria
Inclusion criteria include the following: (1) patients referred to CABG treatment (as assessed by ‘Conventional Heart Team’) having at the time of the ‘Conventional Heart Team’ evaluation at least one de novo stenotic lesion (with a visually assessed DS with ≥50%) in all three major epicardial territories (left anterior descending (LAD) and/or side branch, left circumflex artery (LCX) and/or side branch, right coronary artery (RCA) and/or side branch) supplying viable myocardium with or without left main involvement; (2) patients with hypoplastic RCA with the absence of descending posterior and presence of a lesion in the LAD and LCX territories may be included in the trial as three-vessel disease equivalent. Ostial LAD plus ostial LCX may be included in the trial as a left main equivalent; (3) distal vessel size should be at least 1.5 mm in diameter as visually assessed in the diagnostic angiogram (as requested by the surgeons); (4) patients with silent ischaemia, CCS or stabilised acute coronary syndrome with normalised (stable or decreasing) cardiac biomarker values. For patients showing elevated cardiac troponin (eg, non-ST elevation MI patients) at baseline (within 24 hours pre-CABG) an additional blood sample must be collected prior to CABG to confirm that: (a) high-sensitivity cardiac troponin (hs-cTn) or troponin I or T levels are stable, that is, the value should be within 20% range of the value found in the first sample at baseline, or have dropped23; (b) creatine kinase-muscle/brain (CK-MB) and CK levels are within normal range. If hs-cTn or troponin I or T levels are stable or have dropped, or the CK-MB and CK levels are within normal ranges and the ECG is normal, patients may be included in the study; (5) all anatomical SYNTAX Scores are eligible; (6) patients are amenable to CCTA (eg, no claustrophobia, high heart rate not amenable to beta-blockers, poor renal function, etc., up to the discretion of the investigator); (7) patients have been informed of the nature of the study and agree to its provisions and have provided written informed consent as approved by the Ethical Committee of the respective clinical site; (8) patients agree to 1 month follow-up visit including CCTA.
Exclusion criteria
Candidates will be ineligible for enrolment in the study if any of the following conditions apply: (1) under the age of 18 years; (2) unable to give informed consent; (3) known pregnancy at the time of enrolment; female of childbearing potential, that is, who are not surgically sterile or post-menopausal (defined as no menses for 2 years without an alternative cause); female who is breastfeeding at time of enrolment; (4) prior PCI or CABG; history of coronary stent implantation; (5) evidence of evolving or ongoing ST-elevation MI on ECG and/or elevated cardiac biomarkers (according to local standard hospital practice) have not returned within normal limits at the time of enrolment (non-ST elevation MI); (6) known renal insufficiency (eg, serum creatinine >2.5 mg/dL, or creatinine clearance ≤30 mL/min), or need for dialysis, or acute kidney failure (as per physician judgement); (7) concomitant cardiac valve disease requiring surgical therapy (repair or replacement) and/or aneurysmectomy; (8) single or two-vessel disease (at time of the conventional heart team consensus); (9) non-graftable distal bed in >1 vessel as assessed by the surgeon based on ICA; (10) persistent atrial fibrillation or significant arrhythmias; (11) known allergy to iodinated contrast; (12) a body mass index of 35 or greater; (13) currently participating in another clinical trial not yet at its primary endpoint.
CCTA acquisition
Initial diagnostic screening for assessment of multivessel disease with or without left main involvement may be performed by either ICA or CCTA (if standard of care). Whenever CCTA is not available or when the quality of the initial diagnostic CCTA is suboptimal, CCTA must be obtained/repeated using the 256-slice GE Healthcare Revolution CT scanner and the acquisition should be optimal according to acquisition protocol (online supplemental file). In case a patient had already undergone a GE Healthcare Revolution CT for clinical reasons prior to study start (ie, within 1 month prior to enrolment) but without scanning of the origin and proximal part of mammary arteries (left internal mammary artery/right internal mammary artery), additional CCTA imaging of the mammary arteries could be performed according to the request of the surgeon and according to local practice, to either: (a) add a dedicated small scan for the mammary arteries (if the previous ‘clinical’ CCTA has been obtained with GE Healthcare Revolution CT and is acquired according to the acquisition protocol) or (b) redo the entire scan, that is, mammary arteries plus coronary arteries (in case the image quality of the previous scan is not adequate according to the acquisition protocol). A patient who does not have an evaluable CCTA at baseline (according to the Academic Core Laboratory assessment) will be defined as a ‘screen failure’.
Academic core laboratory
The reconstructed CCTA images should be transferred electronically to the independent Academic Core Laboratory and to HeartFlow (Redwood City, California, USA) for FFRCT. CCTA imaging data will also be provided to GE Healthcare for general assessment of CT exams. Sites may store raw CCTA data for a period of 5 years for additional exploratory analysis ensuring confidentiality of personal data. The anatomical SYNTAX Score by CCTA will be performed by the Academic Core Laboratory. The heart team will consult the Academic Core Laboratory SYNTAX Score during the ‘CCTA Planning and Operating Heart Team’ meeting whenever deemed necessary. The ‘CCTA Planning and Operating Heart Team’ will also receive, from the Academic Core Laboratory for consultation, the SYNTAX Score III that is the combination of the anatomic SYNTAX Score, functional SYNTAX Score and comorbidities (figures 2 and 3). If the Academic Core Laboratory judges that the quality of the CCTA is not sufficient, the CT images may be reconstructed and sent again for assessment of quality.
Figure 2.
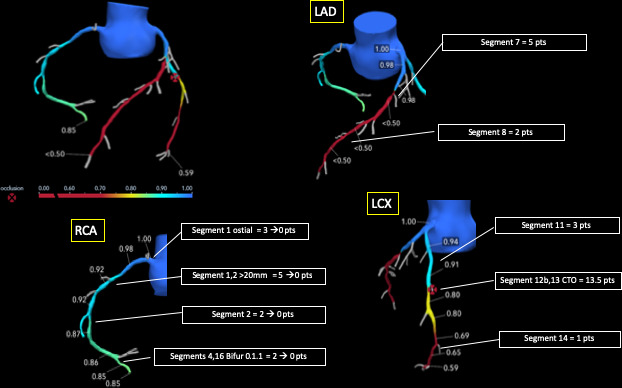
Calculation of functional SYNTAX Score. The functional SYNTAX Score uses the principle of the functional assessment of coronary lesions derived from FFRCT, as is undertaken in conventional anatomical SYNTAX Score calculation. If intermediate stenotic lesions are identified anatomically, but not confirmed as functionally significant, the weighted score of that anatomic segment is subtracted from the functional SYNTAX Score which is combined with the clinical characteristics and comorbidities of the patients to generate the SYNTAX Score III (an integrated indicator of the risk and benefit of revascularisation). SYNTAX Score: Synergy Between percutaneous coronary intervention With Taxus and Cardiac Surgery Score, FFRCT: fractional flow reserve derived from CT angiography; LAD, left anterior descending; LCX, left circumflex artery; RCA, right coronary artery.
Figure 3.
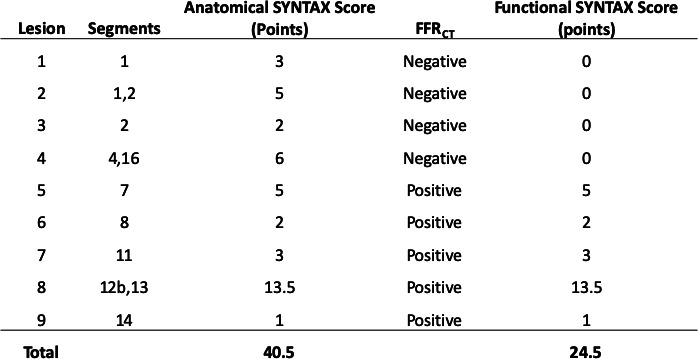
Anatomic SYNTAX Score and functional SYNTAX Score. In this case, anatomical SYNTAX Score is 40.5 point and functional SYNTAX Score is 24.5 points after the calculation (subtraction of the non-physiological stenotic vessel segment with the evaluation of FFRCT). FFRCT, fractional flow reserve derived from CT angiography; SYNTAX Score: Synergy Between percutaneous coronary intervention With Taxus and Cardiac Surgery Score.
Heart team
The ‘Conventional Heart Team’ consists of at least one interventional cardiologist and one cardiac surgeon (figure 1). The ‘Conventional Heart Team’ can be located and operational inside (internal referral) or outside (external referral) the investigational sites. The ‘Conventional Heart Team’ is fully independent of the ‘CCTA Planning and Operating Heart Team’ and study staff, and the clinical contribution of its members will be acknowledged in the appendix of future publications. Planning of the CABG procedure during ‘Conventional Heart Team’ based on ICA will be performed by a cardiac surgeon, blind to the CCTA result and not involved in the ‘CCTA Planning and Operating Heart Team’. The ‘CCTA Planning and Operating Heart Team’ consists of a cardiac surgeon, a radiologist (experienced CCTA reader) and a research nurse/study coordinator (figure 1). It is essential that the ‘CCTA Planning and Operating Heart Team’, who is judging the CCTA, is blind to ICA.
CCTA planning and operating heart team: structure and organisation
The ‘CCTA Planning and Operating Heart Team’ meeting should only start when all information is available and accessible, that is, anatomic SYNTAX Score (by site and by Academic Core Laboratory), and FFRCT. The investigator must confirm in the electronic case report form (eCRF) that all scores/reports (gender, age, peripheral vessel disease, chronic obstructive pulmonary disease, creatinine clearance and anatomical and functional SYNTAX Score) have been reviewed prior to ‘CCTA Planning and Operating Heart Team’ meeting. The CABG procedure has to be executed by the cardiac surgeon or one of his delegated collaborators who have participated in the ‘CCTA Planning and Operating Heart Team’ or are fully informed about the surgical planning of the ‘CCTA Planning and Operating Heart Team’. Any deviation from the surgical planning has to be documented in the eCRF.
Preparation of CABG treatment by means of CCTA
The radiologist (or experienced CCTA reader) of the ‘CCTA Planning and Operating Heart Team’ meeting will confirm the anatomical SYNTAX Score. As an external reference, the independent Academic Core Laboratory also provides anatomical SYNTAX Score on CCTA for consultation and calculates the functional SYNTAX Score by subtracting from the anatomic SYNTAX Score weighted, points of non-flow limiting lesions (>0.80), normally attributed to the weighted automatic lesions (incremental value FFRCT)14 and SYNTAX Score III (=merging of functional SYNTAX Score with comorbidities).
CABG procedure
The CABG procedure will be performed according to local standards.24 Post-CABG, the following data will be collected in the eCRF (actual treatment): (a) number of diseased vessels/diseased lesions; (b) diseased segment number; (c) number and location of anastomoses; (d) type of bypass graft (mammary artery, radial, saphenous vein, other) (online supplemental file, online supplemental video 1); (e) type of grafting: end-to-side (end of the conduit graft to the side of the coronary artery) or side-to-side (sequential, jump graft, or Y-graft). In addition to the actual treatment, only limited details about the CABG procedure will be collected in the eCRF (eg, adjunctive pharmacological treatment, total operation time, clamping time, procedural details (off-pump or on-pump procedure), type of cardioplegia and procedural complications according to the protocol of the EXCEL study (online supplemental file).25
bmjopen-2020-038152supp002.mp4 (874.4KB, mp4)
Follow-up
Patient will undergo a CCTA 30 days (±7 days) after CABG in order to judge the patency and adequacy of revascularisation.26 The follow-up CCTA will be sent to the Academic Core Laboratory as instructed for analysis. An assessment of the anginal status, cardiovascular drug use and any SAE will be recorded, moreover, a standard 12-lead ECG will be performed at the 30 days follow-up visit. These information will be collected in the eCRF.
Patients and public involvement
The FASTTRACK CABG trial is a feasibility study without powered clinical/imaging endpoint. If this feasibility study is positive, then subsequent studies on this topic will be planned to demonstrate the clinical and economical benefit. Due to the nature of the feasibility study, involvement of patient and public is limited to sharing the results of the study after completion of study follow-up.
Ethics and dissemination
Ethical approvals from the ethics committee of the Centro Cardiologico Monzino (R1158/20-CCM 1220), University Hospital of Brussels (B1432020000236) and University Hospital of Jena (2020-1889-1-BO) have been obtained. Each patient has to provide written informed consent as approved by the ethical committee of the respective clinical site. Results will be submitted for publication in peer-reviewed journals and will be disseminated at scientific conferences.
Supplementary Material
Footnotes
Contributors: GP, DA, YO and PWS conceived the core idea of the study. All authors were involved in refining and finalising the study design. HK drafted the manuscript with support from YO and PWS. HK, YO and PWS participated in the statistics. All authors contributed to the critical revision of the manuscript. The corresponding author had the final responsibility for the decision to submit the study publication.
Funding: The FASTTRACK CABG trial is an investigator-driven study sponsored by the National University of Ireland, Galway (NUIG). For this study, the NUIG received research grants from GE Healthcare and HeartFlow.
Competing interests: GWS reports personal fees from Terumo, personal fees from Amaranth, personal fees from Shockwave, personal fees from Valfix, personal fees from TherOx, personal fees from Reva, personal fees from Vascular Dynamics, personal fees from Robocath, personal fees from HeartFlow, personal fees from Gore, personal fees from Ablative Solutions, personal fees from Matrizyme, personal fees from Miracor, personal fees from Neovasc, personal fees from V-wave, personal fees from Abiomed, personal fees from Claret, personal fees from Sirtex, personal fees and other from Ancora, personal fees and other from Qool Therapeutics, other from Cagent, other from Applied Therapeutics, other from Biostar family of funds, other from MedFocus family of funds, personal fees and other from SpectraWave, personal fees from MAIA Pharmaceuticals, personal fees and other from Orchestra Biomed, other from Aria, outside the submitted work. JL is a consultant to and holds stock options in HeartFlow and Circle CVI and receives research grant support from GE Healthcare. BT is an employee of GE Healthcare. CT and CR report personal fees from HeartFlow, Inc, during the conduct of the study. PWS reports personal fees from Biosensors, Medtronic, Micel Technologies, Sinomedical Sciences Technology, St. Jude Medical, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. All other authors have no conflict of interest to declare.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Yusuf S, Zucker D, Peduzzi P, et al. . Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the coronary artery bypass graft surgery Trialists collaboration. Lancet 1994;344:563–70. 10.1016/S0140-6736(94)91963-1 [DOI] [PubMed] [Google Scholar]
- 2.Farooq V, van Klaveren D, Steyerberg EW, et al. . Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013;381:639–50. 10.1016/S0140-6736(13)60108-7 [DOI] [PubMed] [Google Scholar]
- 3.Sianos G, Morel M-A, Kappetein AP, et al. . The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–27. [PubMed] [Google Scholar]
- 4.Serruys PW, Chichareon P, Modolo R, et al. . The SYNTAX score on its way out or…towards artificial intelligence: Part I. EuroIntervention 2019. [Google Scholar]
- 5.Serruys PW, Chichareon P, Modolo R, et al. . The SYNTAX score on its way out or…towards artificial intelligence: Part II. EuroIntervention 2019. [Google Scholar]
- 6.Collet C, Onuma Y, Andreini D, et al. . Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J 2018;39:3689–98. 10.1093/eurheartj/ehy581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onuma Y, Tanabe K, Chihara R, et al. . Evaluation of coronary artery bypass grafts and native coronary arteries using 64-slice multidetector computed tomography. Am Heart J 2007;154:519–26. 10.1016/j.ahj.2007.04.054 [DOI] [PubMed] [Google Scholar]
- 8.Collet C, Onuma Y, Grundeken MJ, et al. . In vitro validation of coronary CT angiography for the evaluation of complex lesions. EuroIntervention 2018;13:e1823–30. 10.4244/EIJ-D-17-00326 [DOI] [PubMed] [Google Scholar]
- 9.Douglas PS, Hoffmann U, Patel MR, et al. . Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. 10.1056/NEJMoa1415516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onuma Y, Collet C, van Geuns R-J, et al. . Long-Term serial non-invasive multislice computed tomography angiography with functional evaluation after coronary implantation of a bioresorbable everolimus-eluting scaffold: the absorb cohort B MSCT substudy. Eur Heart J Cardiovasc Imaging 2017;18:870–9. 10.1093/ehjci/jex022 [DOI] [PubMed] [Google Scholar]
- 11.Collet C, Miyazaki Y, Ryan N, et al. . Fractional flow reserve derived from computed tomographic angiography in patients with multivessel CAD. J Am Coll Cardiol 2018;71:2756–69. 10.1016/j.jacc.2018.02.053 [DOI] [PubMed] [Google Scholar]
- 12.Moss AJ, Williams MC, Newby DE, et al. . The updated NICE guidelines: cardiac CT as the first-line test for coronary artery disease. Curr Cardiovasc Imaging Rep 2017;10:15. 10.1007/s12410-017-9412-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knuuti J, Wijns W, et al. , Group ESCSD . Esc guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2019;2019. [DOI] [PubMed] [Google Scholar]
- 14.Nørgaard BL, Leipsic J, Gaur S, et al. . Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol 2014;63:1145–55. 10.1016/j.jacc.2013.11.043 [DOI] [PubMed] [Google Scholar]
- 15.Driessen RS, Danad I, Stuijfzand WJ, et al. . Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol 2019;73:161–73. 10.1016/j.jacc.2018.10.056 [DOI] [PubMed] [Google Scholar]
- 16.Neumann FJ, Sousa-Uva M, et al. , Group ESCSD . ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2018;2019:87–165. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Calhoon JH, Dehmer GJ, et al. . ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;69:2212–41. 10.1016/j.jacc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 18.Sonck J, Miyazaki Y, Collet C, et al. . Feasibility of planning coronary artery bypass grafting based only on coronary computed tomography angiography and CT-derived fractional flow reserve: a pilot survey of the surgeons involved in the randomized SYNTAX III revolution trial. Interact Cardiovasc Thorac Surg 2019 10.1093/icvts/ivz046. [Epub ahead of print: 18 Mar 2019]. [DOI] [PubMed] [Google Scholar]
- 19.Nunnally JC, Bernstein IH. Psychometric theory. (3rd ed.) New York: McGraw-Hill, 1994. [Google Scholar]
- 20.Lees KR, Bath PMW, Schellinger PD, et al. . Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke 2012;43:1163–70. 10.1161/STROKEAHA.111.641423 [DOI] [PubMed] [Google Scholar]
- 21.Kappetein AP, Serruys PW, Sabik JF, et al. . Design and rationale for a randomised comparison of everolimus-eluting stents and coronary artery bypass graft surgery in selected patients with left main coronary artery disease: the EXCEL trial. EuroIntervention 2016;12:861–72. 10.4244/EIJV12I7A141 [DOI] [PubMed] [Google Scholar]
- 22.Melina G, Angeloni E, Refice S, et al. . Residual SYNTAX score following coronary artery bypass grafting. Eur J Cardiothorac Surg 2017;51:547–53. 10.1093/ejcts/ezw356 [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert JS, Jaffe AS, et al. . Fourth universal definition of myocardial infarction (2018). Circulation 2018;138:e618–51. 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 24.Serruys PW, Morice M-C, Kappetein AP, et al. . Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–72. 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 25.Stone GW, Sabik JF, Serruys PW, et al. . 3Rd, Taggart DP, banning a, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogats G, Mansour S, Noiseux N, Sabate M, Pomar J, hickey M, Gershlick a, Buszman P, Bochenek a, Schampaert E, page P, Dressler O, Kosmidou I, Mehran R, Pocock SJ, Kappetein AP, Investigators et. everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016;375:2223–35.27797291 [Google Scholar]
- 26.Mushtaq S, Conte E, Pontone G, et al. . Interpretability of coronary CT angiography performed with a novel whole-heart coverage high-definition CT scanner in 300 consecutive patients with coronary artery bypass grafts. J Cardiovasc Comput Tomogr 2020;14:137–43. 10.1016/j.jcct.2019.08.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038152supp001.pdf (2.2MB, pdf)
bmjopen-2020-038152supp002.mp4 (874.4KB, mp4)


