Scheme 1.
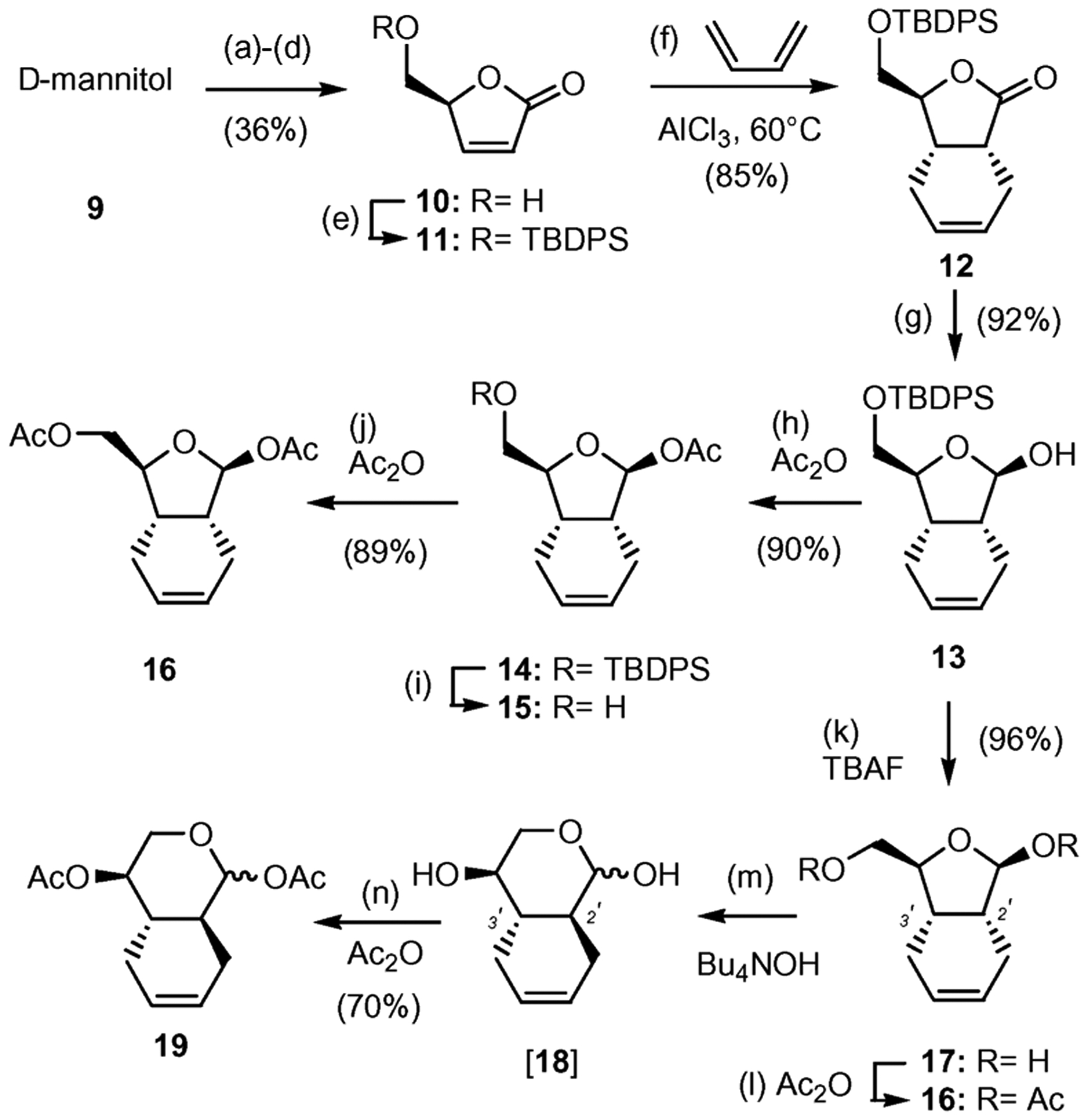
Synthesis of bicyclic scaffold 16. Reagents and conditions: (a) 0.01 equiv SnCl2, 2,2-dimethoxypropane–DME (2 : 3), reflux, 4 h, 78%; (b) 2.0 equiv NaIO4, sat. aq. NaHCO3–CH2Cl2 (1 : 20), 0 °C, 2 h, 73%; (c) 1.5 equiv Ph3P=CHCO2Me, MeOH, 0 °C, 16 h, 70%; (d) conc. H2SO4–MeOH (1 : 100), 25 °C, 1 h, 90% (e) 1.2 equiv TBDPSCl, 5.0 equiv NH4NO3, DMF, 25 ° (f) 0.33 equiv AlCl3, CH2Cl2, 60 °C, 6 d, 85%; (g) 1.1 equiv DIBAL-H (1 M), CH2Cl2, −78 °C 1 h, 92%; (h) 3.0 equiv Ac2O, pyridine, 25 °C, 12 h, 90%; (i) 1.2 equiv TBAF (1 M), THF, 25 °C, 30 min, 87%; (j) 3.0 equiv Ac2O, pyridine, 25 °C, 12 h, 89%; (k) 1.2 equiv TBAF (1 M), THF, 25 °C, 25 min, 96%; (l) 4.0 equiv Ac2O, pyridine, 25 °C, 12 h, 92%; (m) 1.3 equiv Bu4NOH (33% in H2O), THF, 25 °C, 6 h; (n) 4.0 equiv of Ac2O, pyridine, 25 °C, 12 h, 70% (over two steps).
