Figure 2.
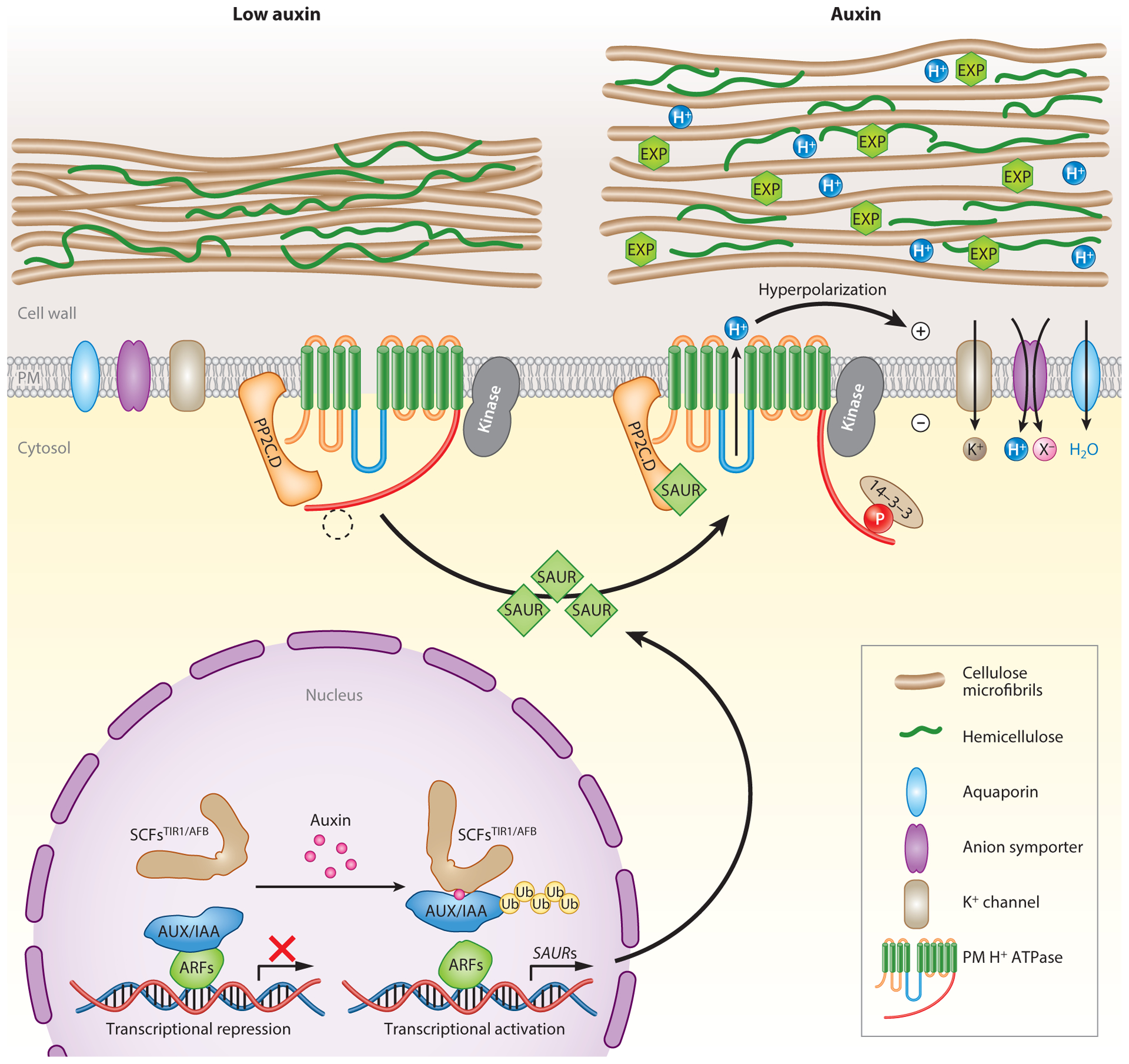
A model for auxin-mediated acid growth. Under low auxin conditions (left), basal plasma membrane (PM) H+-ATPase activity is maintained through the balance of phosphorylation via an unknown protein kinase and dephosphorylation by PP2C.D protein phosphatases. In response to auxin (right), canonical SCFTIR1/AFB-mediated signaling promotes SAUR gene expression. Following protein synthesis, SAUR proteins directly interact with and inhibit PM-localized PP2C.D2/PP2C.D5/PP2C.D6, preventing these phosphatases from dephosphorylating Thr947 and perhaps additional residues of PM H+-ATPases, thus keeping these proton pumps in the phosphorylated, activated state. This increase in PM H+-ATPase activity acidifies the apoplast, activating expansins (EXPs) and cell wall-remodeling enzymes to loosen the cell wall. Proton pumping also hyperpolarizes the PM, which activates inward-rectifying K+ channels and energizes H+-coupled anion symporters (X−). These transport activities maintain solute uptake needed for sustained water uptake and turgor pressure maintenance.
