Rheumatology key message
Heterozygous as well as homozygous mutations of SLC22A12 can cause renal hypouricaemia with its complications.
Dear Editor, We herein report two Japanese families with inherited renal hypouricaemia (RHUC) associated with a functionally null variant in an exon–intron boundary of the urate transporter 1 (URAT1, also known as SLC22A12) gene. URAT1 dysfunction is reported to cause RHUC type 1 [1, 2] and several variants of URAT1 have also been reported to be associated with serum uric acid (SUA) level [3, 4]. Glucose transporter 9 (GLUT9, also known as SLC2A9) dysfunction is reported to cause RHUC type 2 [5, 6]. This inherited and heterogeneous disorder is characterized by low SUA levels [≤2 mg/dl or 120 µM (normal range 3.0–7.0 mg/dl)] resulting from increased renal urate excretion due to insufficient urate reabsorption: it causes severe complications such as exercise-induced acute kidney injury and urolithiasis [7]. However, we found some patients who have no exonic mutations in URAT1 that cause RHUC. We consider this study to be the first report of a URAT1 intronic variant as an aetiologic factor for RHUC.
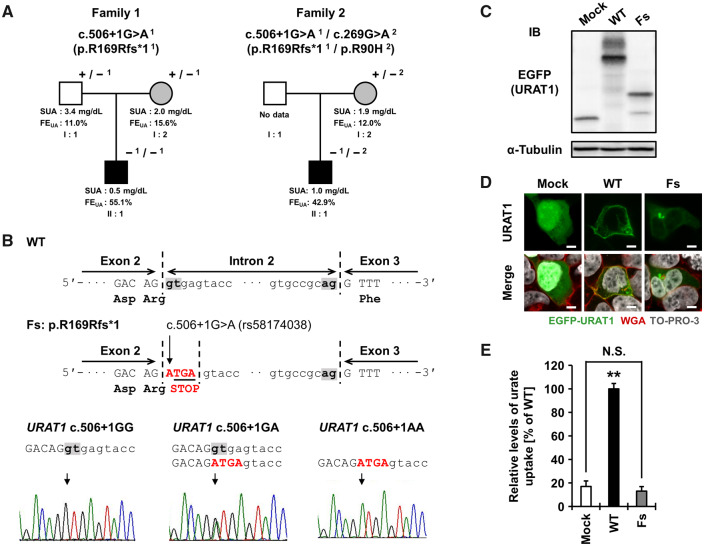
The pedigrees are described in Fig. 1A. Patient II:1 in family 1 and 2 exhibited extremely low levels of SUA (0.5 mg/dl and 1.0 mg/dl, respectively) and a markedly high level of fractional excretion of uric acid [FEUA, 55.1% and 42.9%, respectively (normal range 5.5–11.1%)], which are typical features of RHUC. These results suggest that dysfunctional variants of URAT1 or GLUT9 could be involved in the RHUC seen in our cases. Detailed information on subjects are available in the supplementary note and Table S1, available at Rheumatology online. This study was approved by the institutional ethical committees. Written consent was obtained from all participants. All procedures involved were performed in accordance with the Declaration of Helsinki.
Fig. 1.
Identification and functional validation for c.506 + 1G>A of URAT1 in two families with renal hypouricaemia (RHUC)
(A) Two families with RHUC. +, wild-type allele; −1, mutant allele of c.506 + 1G>A (rs58174038); −2, mutant allele of R90H. White, unaffected subject; grey, subjects with a mutant allele; black, subjects with two mutant alleles. (B) The position and representative sequences of c.506 + 1G>A in URAT1. WT, wild-type; Fs, frameshift. (C) Immunoblot detection of URAT1 protein from transfected 293A cells. (D) Confocal microscopic observations of URAT1 protein. Bars, 5 µm. (E) Urate transport activities by cell-based transport assay. **P < 0.01 vs the other groups; N.S., not significant. Detailed information for Fig. 1 is described in supplementary material, available at Rheumatology online.
To explore the possible causes of these two familial RHUC cases, we conducted genetic analyses targeting URAT1. Direct sequencing was initially performed for these cases to seek W258X and R90H in URAT1—the first and second most frequent dysfunctional mutations that cause RHUC in the Japanese population. However, we detected only a heterozygous R90H mutation in family 2 and no mutations in family 1. Direct sequencing of all exons of URAT1 was next performed as shown in supplementary methods and Table S2, available at Rheumatology online, which identified an intronic URAT1 variant (rs58174038, c.506 + 1G>A) in the boundary region between exon 2 and intron 2 in both families (Fig. 1A and B).
Patient II:1 of family 1 with RHUC presented homozygous mutations, indicating that two alleles of this variant are demonstrably related to the development of RHUC. Notably, subject I:2 (the proband’s mother; c.506 + 1G>A heterozygote) of family 1 (SUA 2.0 mg/dl, FEUA 15.6%) also met the diagnostic criteria for RHUC (SUA ≤2.0 mg/dl) [7] and had a past history of urolithiasis. Whereas subject I:1 (the proband’s father; c.506 + 1G>A heterozygote) did not meet the diagnostic criteria for RHUC, the effect of one allele of this splicing mutation was suggested by the slightly lowered SUA for men (3.4 mg/dl) and mild elevation of FEUA (11.0%).
Patient II:1 of family 2 had a non-synonymous variant (R90H), reported as a functionally null mutation [2], in addition to a variant of c.506 + 1G>A (Fig. 1A). When compared with those of his mother (patient I:2) having only a heterozygous R90H in URAT1 (SUA 1.9 mg/dl, FEUA 12.0%), patient II:1 (the compound heterozygote of c.506 + 1G>A and R90H) exhibited severely low SUA (1.0 mg/dl) and ∼3.5-fold higher FEUA (42.9%). It is therefore probable that URAT1 c.506 + 1G>A is responsible for this familial RHUC due to the disruption of URAT1’s function as a urate reabsorption transporter.
We therefore performed functional validation using cell-based assays [8] to examine the effects of c.506 + 1G>A (supplementary methods and Table S3, available at Rheumatology online). This variant disrupts the original splice donor site in intron 2 of URAT1 (Fig. 1B), which appeared to result in the production of a premature stop codon (p.R169Rfs*1). We constructed the expression vector for this frameshift variant using a site-directed mutagenesis technique from pEGFP-C1/URAT1 wild-type plasmid for EGFP-URAT1 expression as a starting material. First, we performed immunoblot analysis using an anti-EGFP antibody to detect EGFP-tagged URAT1 (Fig. 1C). As expected, unlike the URAT1 wild-type, the frameshift variant was expressed as a truncated form. Next, confocal microscopy revealed that under our experimental conditions, URAT1 wild-type was localized on the plasma membrane, while the frameshift variant was rarely observed on the cell surface (Fig. 1D). Finally, our urate transport assay confirmed the frameshift variant to be functionally null (Fig. 1E).
Considering the following three points together with the renal expression pattern of URAT1 [1], we conclude that this splicing mutation in URAT1 is responsible for RHUC. First, this splicing mutation in URAT1 caused almost null function as a urate reabsorption transporter (Fig. 1E). Second, patients with this dysfunctional variant had increased FEUA levels and decreased SUA levels. Third, clinical genetic analyses of the two families with RHUC with this variant revealed consistent results.
In summary, we have identified a functionally null intronic mutation of URAT1 that causes RHUC in two Japanese pedigrees. These findings contribute to a better understanding of the genetic aetiology of RHUC.
Supplementary Material
Acknowledgements
We would like to thank all the participants for their generous involvement in this study. We are indebted to M. Miyazawa and K. Morichika (National Defence Medical College) for genetic analysis. We are grateful to H. Ueda (Osaka City General Hospital) for sample collection and clinical analysis. Y.K., Y.T., T.H., T.T. and H.M. conceived and designed the study. T.O., R.H., M.Y., I.K., R.F. and H.M. analysed the clinical data of the cases. Y.T. and T.T. performed functional analyses. Y.K., T.H., A.N., S.S. and H.M. performed the genetic analyses. H.S. and N.S. provided intellectual input and assisted with the preparation of the manuscript. Y.K., Y.T., A.N., T.T. and H.M. wrote the manuscript. H.M. organized clinico-genetic analysis and T.T. supervised functional analysis for our paper.
Funding: This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, including MEXT Kakenhi (17H04128 and 19K22786) and JSPS Kakenhi grants (16H01808, 18KK0247, 19K16441, 20H00566 and 20H00568), The Uehara Memorial Foundation, Mochida Science Foundation for Medical and Pharmaceutical Research, Takeda Medical Foundation, MSD Life Science Foundation, Public Interest Incorporated Foundation, Kawano Masanori Memorial Foundation for Promotion of Pediatrics and the Gout and Uric Acid Foundation of Japan.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Enomoto A, Kimura H, Chairoungdua A. et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002;417:447–52. [DOI] [PubMed] [Google Scholar]
- 2. Ichida K, Hosoyamada M, Hisatome I. et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J Am Soc Nephrol 2004;15:164–73. [DOI] [PubMed] [Google Scholar]
- 3. Nakatochi M, Kanai M, Nakayama A. et al. Genome-wide meta-analysis identifies multiple novel loci associated with serum uric acid levels in Japanese individuals. Commun Biol 2019;2:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Misawa K, Hasegawa T, Mishima E. et al. Contribution of rare variants of the SLC22A12 gene to the missing heritability of serum urate levels. Genetics 2020;214: 1079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsuo H, Chiba T, Nagamori S. et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet 2008;83:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dinour D, Gray NK, Campbell S. et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol 2010;21:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakayama A, Matsuo H, Ohtahara A. et al. Clinical practice guideline for renal hypouricemia (1st edition). Hum Cell 2019;32:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyata H, Takada T, Toyoda Y. et al. Identification of Febuxostat as a new strong ABCG2 inhibitor: potential applications and risks in clinical situations. Front Pharmacol 2016;7:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.