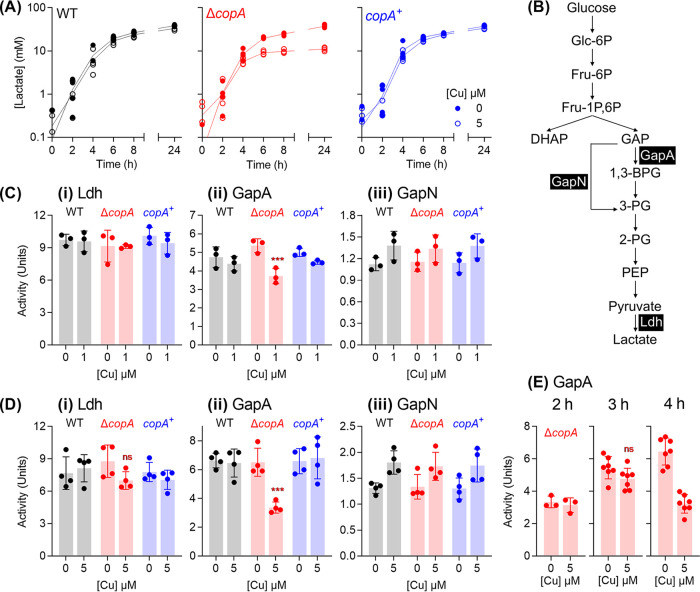
FIG 3.
Cu-dependent defects in glycolysis and homolactic fermentation. (A) Lactate production. GAS strains were cultured with added Cu as indicated (n = 3). Amounts of lactate secreted to the extracellular culture medium were measured at the indicated time points. Cu treatment suppressed lactate production in the ΔcopA cultures (P < 0.0001). (B) Fermentation pathway in GAS. Enzymes of interest, namely, GapA (NAD+-dependent GAPDH, M5005_SPy_0233), GapN (NADP+-dependent GAPDH, M5005_SPy_1119), and Ldh (lactate dehydrogenase, M5005_SPy_0873) are shown. (C and D) Activity of glycolytic enzymes Ldh (i), GapA (ii), and GapN (iii). GAS strains were cultured for t = 4 h with 0 or 1 μM added Cu (n = 3) (C) or 0 or 5 μM added Cu (n = 4) (D). Enzyme activities were determined in cell extracts. Cu treatment decreased GapA activity in ΔcopA cultures (***, P = 0.0004). ns, P = 0.14. (E) GapA activity over time. GAS ΔcopA mutant strain was cultured with added Cu as indicated for t = 2 h (n = 3), 3 h (n = 7), or 4 h (n = 7). Enzyme activities were determined in cell extracts. Cu treatment did not have an effect on GapA activity at t = 2 h (P = 0.99) or 3 h (ns, P = 0.18), but it strongly inhibited GapA activity at t = 4 h (P < 0.0001). All statistical analyses were versus 0 μM Cu.